Abstract
Bitter vetch (Vicia ervilia (L.) Willd.), one of the Near Eastern founder crops, is an annual cleistogamous legume domesticated during the Neolithic period. Originally used for human consumption, over time it was replaced by other pulses and downgraded to a fodder crop. When coupled with a small degree of cross hybridization, cleistogamy confers evolutive plasticity to the plant species. The aim of the present work consisted in setting up optimal conditions to overcome the existing cross hybridization barriers in V. ervilia. Genotypes of Turkish origin, characterized by an erect growth habit were crossed with Italian counterparts characterized by high seed production. A detailed cyto-histological analysis of flower development was undertaken to determine the optimal stage for emasculation and manual cross. Ninety-eight crosses were carried out and the hybrid nature of the putative F1 progenies was assessed by SSR (simple sequence repeat) DNA markers. Fifty-five seeds were obtained of which only five gave rise to hybrid plants. Among these, only three turned out to be fertile and two of which generated a consistent number of F2 seeds whose plants were assessed in greenhouse for seed production and plant growth habit. Most of the evaluated traits showed mean values of the F2 plants intermediate between the two initial parents, confirming that intraspecific hybridization is not only possible but also useful to exploit the diversity confined in different bitter vetch populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The speciation process is due to the development of reproductive isolation barriers that maintain the species integrity by restricting the gene flow between clustered phenotypes (Christie and Strauss 2019). External barriers to genetic interchange between related populations prevent plant pollen of one population from falling on plant stigmas of the other population. Among these barriers, cleistogamy, reported across 228 genera and 50 families (Culley and Klooster 2007), is able to develop receptive stigma and viable pollen shed in closed flowers (Lord 1981). Cleistogamous populations detain only maternal information and tend to preserve locally adapted genes (Schoen and Lloyd 1984; Winn and Moriuchi 2009) but, on the other hand, they increase their genetic drift and inbreeding depression if deleterious mutations cannot be purged (Lloyd 1979; Lande and Schemske 1985). When coupled with cross-pollination (chasmogamy), cleistogamy confers an overall reproductive plasticity leading to a fitness advantage in species adopting a mixed-mating strategy (Koontz et al. 2017). In these species, genetic engineering of strict cleistogamous genotypes may represent a useful strategy for bioconfinement of transgenic pollen (Huang et al. 2023). Unfortunately, cleistogamy represents a major bottleneck to the introgression of agronomic traits of interest into crops through hybridization with wild germplasm (Hadley and Openshaw 1980).
Bitter vetch (Vicia ervilia (L.) Willd.), one of the Near East founder crops, is an annual cleistogamous legume domesticated during the Neolithic period (Ladizinsky 1998). Although originally used for human consumption, over the years this species has become a fodder crop because other pulses took place as preferred food. Today, although being highly tolerant to drought and cold, it is an underutilized species, rarely used in rotation regimes in low input farming systems (Ratinam et al. 1994; Ates et al. 2014). On the other hand, as a leguminous species, cultivated bitter vetch is able to return reduced nitrogen to the soil and holding non-shattering pods, it can be easily harvested at complete seed maturity by uprooting with appropriate lifters (Esteban 1996). The seeds, used in the past as protein source in animal feed formulations, nowadays are replaced by other protein sources, leading to a EU dependency on imported soybean (Sun et al. 2018). This gives rise to the EU necessity of exploiting alternative protein sources into new as well as existing processes or products, thus developing and ensuring more sustainable and resilient supply chains (Verstringe et al. 2023). Recent advances in animal feeding trials have yielded encouraging evidences on the use of bitter vetch seeds in animal feeding (Russi et al. 2019). Besides, protein seed extracts from V. ervilia have been successfully used to produce environmentally friendly edible films for food packaging (Porta et al. 2017). In summary, thanks to the plasticity of use, bitter vetch can be considered an interesting and versatile alternative crop for its re-introduction in marginal, abandoned areas of Mediterranean basin where plant cultivation for human consumption is not economically convenient (Ordóñez-Fernández et al. 2018; Berger et al. 2002). However, due to lodging problems and creeping habitus of bitter vetch Italian germplasm, a considerable number of seeds is lost during mechanical harvesting. One possible way to circumvent these yield losses is to breed and transfer the erect growth habit trait to high seed yielding genotypes. Negative correlations are reported by Livanios et al. (2018) between plant high and lodging susceptibility, whereas seed yield correlated positively with prostrate growth habitus and, as a consequence, with plant high (Russi et al. 2019). Unfortunately, no genotype with erect habitus has been detected among high seed yielding landraces adapted to Central Italy, whereas many erect genotypes are found among landraces collected in Turkey (Russi et al. 2019). Therefore, it would be of great interest to improve seed yields by combining the high seed yield trait, available in locally adapted Italian germplasm, with the erect growth habitus from Turkish landraces. Even in reported cases of bitter vetch plants originated from artificial cross pollination (Ladizinsky and van Oss 1984; Zhang and Mosjidis 1995), no solid evidence of hybridity by using molecular or biochemical markers has been described. Therefore, the overall objective of the present work is to set up a protocol to overcome the existing cross hybridization barriers among V. ervilia genotypes, facilitating gene transfer from wild germplasm to adapted genotypes in future breeding programs.
Materials and methods
Plant materials and growth conditions
Twenty seeds per accession of V. ervilia were sown in the greenhouse on February 1st 2022 in pots 20 × 20 cm containing a mixture of soil and peat (1:1). Table 1 reports the parents from the Turkish accessions 8, 10 and 14, all characterized by an erect growth habit (GH), and the Italian accessions 21, 22 and 23, adapted to Central Italy and characterized by prostrate GH (Russi et al 2019). Ten days later, additional 20 seeds per accession were sown to obtain a scalar flowering time in order to enhance the probabilities of synchronized flowering between erect and prostrate parental lines. All plants were grown under routine practices in greenhouses with temperatures maintained at 24–28 °C (Russi et al. 2019). Crosses were carried out during May/June 2022 at 100% relative humidity to avoid self-fertilization because under these environmental conditions the swollen pollen of the mother plant could not get out of the anthers. Seeds of parental lines and putative F1 hybrids were sown in September 2022 and analyzed for SSR markers (see the Molecular analysis section) in November 2022. F2 seeds from confirmed F1 hybrids were sown in March/April 2023, assessed for dry matter yield and seed production, and harvested from June to September 2023.
Histological analyses of gametophyte development
In order to successfully perform crosses between different accessions, the optimal stage of V. ervilia flower development had to be established. Both the stigma receptivity before fertilization by pollen of the same flower and the optimal degree of pollen maturation were analyzed, using three plants per accession and 30–50 flowers per plant. Flowers were harvested at different stages of development and fixed in a mixture of absolute ethanol-acetic acid 3:1 (v/v). During the preparation of histological sections, fixed samples were dehydrated in a series of ethanol solutions. After one hour, ethanol was replaced with xylene through successive passages in xylene-ethanol mixtures with increasing xylene concentration. The next day, the material was embedded in paraffin in the same way, by successive passages in xylene-paraffin mixtures with increasing concentration of paraffin maintained at 57 °C, until 100% paraffin was reached. Flowers were cut by a microtome into 12 µm sections. Sections were double-stained with Safranin and Fast Green mixture. Briefly, sample hydration was carried out by serial transfers in ethanol–water mixtures with decreasing ethanol concentration from 95 to 70%. After applying Safranin, dehydration was achieved returning to a concentration of 90% ethanol. At this point, the addition of Fast Green was followed by two passages in absolute ethanol and by other two passages in pure xylene. Sections were sealed with a coverslip fixed with DPX Mountant for histology and samples were observed under a light microscope (DMRB, Leica).
Pollen viability
Three flowers per plant of each accession were collected. Mature pollen was distributed on slides and stained with a 1:1 acetocarmine:glycerol solution (v/v) (Akaffou et al. 2014). Three to four hundred pollen grains per plant were analyzed at the microscope. Rounded, red-colored pollen grains were judged to be viable, while pale red or yellow ones were considered as non-viable.
Cross procedures
A variable number of flowers per accessions were chosen for emasculation when the petals reached three-quarters of the sepal length (see Results). The anthers were removed by forceps and stigmas were manually pollinated. In order to prevent self-fertilization or damages to the stigma, special care was taken during emasculation to avoid any contact between the stigma and the anthers of the same flower. The pollinated flowers were wrapped in a labeled paper bag until seed maturation.
Molecular analysis
Genomic DNA was isolated from 100 mg (fresh weight) of young and healthy leaves, using the Gene Elute™ Plant Genomic DNA miniprep Kit (Merck, Darmstadt, Germany) according to the manufacturer’s instructions. The primers for the four SSR loci (VE02, VE05, VE07 and VE09) came from Russi et al. (2019). Primer sequences and PCR conditions were described in El Fatehi et al. (2013). Amplifications were preliminary tested with unlabelled primers to assure that the amplicons were within the range of 100–250 bp. SSR analysis was then performed by using fluorescent-dye-labelled primers, and the resulting PCR products were analysed on an ABI 3130 sequencer using the GeneScan 600 LIZ size as molecular weight standard (Thermo Fisher Scientific). SSR profiles were visualized with the XCR Software.
Statistical analysis
Mean sterile pollen percentages were compared by the Duncan test to a significance level of P ≤ 0.01 using the InfoStat Professional v. 1.1 software (Di Rienzo et al. 2012).
Data on seed yield components (number of pods per plant, number of seeds per pod, seed yield and seed number per plant, 1000-seed-weight), on dry matter yield per plant, on growth habit of the two parents per cross, and on the F2 hybrids were analyzed by ANOVA and means separated by the Student–Newman–Keuls test using the SAS software (SAS Institute).
Results
Cyto-histological analysis of V. ervilia flowers
The cleistogamous nature of V. ervilia (fertilization takes place before the opening of hermaphrodite flower) is a physical barrier which prevents cross-fertilization, hence transfer of important traits in breeding programs. Difficulties in crossing Turkish erect-habitus genotypes with adapted high seed yielding Italian landraces should first be investigated and then overcome. For this purpose, studies on the development of male and female gametophytes were undertaken to seek a correlation of macrogametogenesis and stigma receptivity fulfillment with external morphology and dimension of the flowers.
Overall, male meiosis in bitter vetch preceded megasporogenesis (Fig. 1). Tetrads were frequently observed together with female gametophytes at the beginning of megagametogenesis or at earlier phases in the same flower sections (Fig. 1a). More precisely, female meiosis started when pollen grains had already been binucleated (Fig. 1c). Ovules of the hemianatropous type, usually three in number (Fig. 1b), and penta-cellularized hairs were noticed on the basal stigma and on the apical portion of the stylus (Fig. 1d).
Histological analysis of developing gametophytes and seeds in Vicia ervilia. Anthers at the tetrad stage and ovules before the onset of megasporogenesis a. Hemianatropous ovules b. Beginning of megasporogenesis and uni/bi-nucleated pollen grains in the anthers c. Penta-cellularized hairs on the apical portion of the style (arrows, d). Arrows in the following panels show: the initial archesporial cell e, the megaspore mother cell f, the chalazal megaspores (black, g) and the epichalazal megaspore (white, g), the all four degenerated megaspore of Italian accession 21 (h). Binucleate and tetranucleated embryo sacs are represented in i and j respectively, whereas in k it is reported a mature embryo sac showing an egg cell (white arrow), one sperm cell (white arrowhead) and two polar nuclei (black arrow) that fuse together with a single sperm nucleus giving rise to a cell (arrow, l) precursor of the endosperm. Early and late phases of syncytium formation are reported in m and n, respectively, in which arrows point to syncytial cells layered on a side of the embryo sac. The arrow in o points to the developed embryo with more than four cells and in p to the early endosperm during cellularization. Safranine-fast green staining. Scale bar: a–c = 150 µm, d–k = 20 µm, l–p = 30 µm
Then, we investigated in detail the megasporogenesis and megagametogenesis processes (Fig. 1e–p) in relation to the floral morphogenesis (Fig. 2). Longitudinal sections of hermaphrodite flowers containing a developing ovule showed a multicellular archesporium that differentiates beneath two layers of nucellar cells. One of the archesporium cells increases in volume, showing a dense cytoplasm as well as a nucleolus into the nucleus (Fig. 1e). This cell differentiated into the megaspore mother cell (MMC), compressing the lateral cells and stretching along the major nucellar axis (Fig. 1f). The MMC first meiotic division produced two identical cells; the chalazal one began its second meiotic division before the micropylar cell, resulting in an asynchronous meiosis II that produces a typical linear array of four megaspores. The chalazal megaspore developed as functional megaspore, whereas the epichalazal cell remained active for short time and died after degeneration of the two micropylar megaspores (Fig. 1g). These events occurred in the same way in all the accessions studied. It should be noted that Italian accession 21 showed the highest percentage (5.26%) of ovules with all degenerated megaspores (Fig. 1h). The functional megaspore then underwent three mitotic divisions giving rise to two (Fig. 1i), four (Fig. 1j) and eight-nucleated (not shown) embryo sac of the Polygonum type. The three haploid nuclei at the micropylar end evolved into a group of cells forming the apparatus containing an egg cell (white arrow, Fig. 1k) that is fertilized by a sperm nucleus (arrowhead, Fig. 1k). Although in many angiosperms the three antipodal nuclei evolve into three or more cells, in all accessions of V. ervilia studied here, the antipodal nuclei degenerated earlier without differentiating in cells. The two remaining nuclei (polar nuclei; arrow, Fig. 1k) moved to the center of the rising embryo sac, fuse with a single sperm nucleus giving rise to the precursor cell (triploid) of the endosperm (arrow, Fig. 1l). After the double fertilization has completed, the endosperm development followed a nuclear developmental model. Initially, less than 20 nuclei formed the syncytium (Fig. 1m). After a week, while the syncytial cells lined up (Fig. 1n), the zygote evolved into an eight-cell globular embryo (Fig. 1o). Immediately after the syncytial phase, the developing endosperm reached the cellularization stage (Fig. 1p).
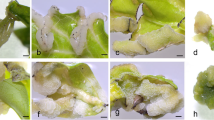
Flowers at different stages of development a–d and corresponding developmental stages of the carpel e–h and ovule (in longitudinal sections, i–l). In preanthesis stage a, e, the megaspore mother cell can be observed in the ovule (arrow, i). At the anthesis (b, f), the mature embryo sac shows the egg apparatus and polar nuclei (white and black arrows respectively, j). At post anthesis stage c and g, the first divisions of the syncytium can be observed (arrows, k). At later post anthesis stage d, h, the syncytium is layered to the sides of the embryo sac and an embryo at early phase of development is present (arrow, l). Safranine-fast green staining i–l. Scale bar: a–h = 1 cm, i–j = 20 µm, k–l = 30 µm
To maximize the probabilities of successful hybridization, we then tried to find the correlation between stigma receptivity and external floral morphology (Fig. 2). When the petals were still completely covered by sepals (Fig. 2a), and the anthers were shorter than the pistil (Fig. 2e), only MMC was present in the ovule (Fig. 2i), so this stage resulted too early for artificial fertilization. When the flower became around 4.8 mm long with sepals still a bit longer than the petals (Fig. 2b),the gynoecium measured 3.2 mm long and the anthers were still closed but reached almost to the same level of the stigma (Fig. 2f), the embryo sac was completely developed (Fig. 2j). Therefore, this stage turned out to be optimal for manual crosses. In later floral development stages (Fig. 2c, d), when the petals were longer than the sepals, pistils tended to curve (Fig. 2g) and the anthers began to degenerate (Fig. 2h), the fertilization had already occurred as endosperm syncytia at initial (Fig. 2k) and advanced stages (more than 20 nuclei) were observed (Fig. 2l).
Moreover, to investigate whether the general low seed set could be caused by pollen viability, mean percentages of vital pollen were assessed. The mean percentage of sterile pollen was low and within the range of 0.32–2.72% in all accessions, except for Italian accession 21, where the percentage resulted significantly higher, 9.06% (Fig. S1).
SSR characterization of the Italian and Turkish parental lines
Once established the optimal flowering stage for crosses, 28 parental plants (15 Turkish and 13 Italian) were selected. Taking into consideration the flowering time difference (Turkish accessions flowered earlier than Italian ones, Russi et al. 2019), seeds were sown at differentiated times (see Materials and methods) in order to synchronize the blooms of both Italian and Turkish accessions. Three to eight plants per accession were analyzed with three informative SSR markers (Table S1). In total, fourteen genotypes were identified of which four for the accessions 10 and 22, three for accession 14 and only one for the accessions 8, 21 and 23 (Table S1). These plants were used as parents in 30 cross combinations, which, considering the parent genotypes, represented 26 different genotype combinations, where the female parent was alternately a Turkish (12 combinations), or an Italian accession (14 combinations, Table S2). The crossing results, summarized in Table 1, showed that 68 out of 98 attempted crosses were unsuccessful and 30 generated at least one pod containing 1–3 seeds per fertilized flower. Fifty-five seeds were obtained, which generated 55 plants whose hybrid or self-fertilization nature was ascertained by SSR analysis (Table S2). Among these, 50 plants showing the maternal genotype were classified as self-fertilization products whereas five plants contained both maternal and paternal alleles confirming their hybrid nature. An example of SSR profile of parental and hybrid pattern is shown in Fig. 3. The two parental lines, 23 and 8 (both of them were represented by a single genotype, Table S1), were polymorphic and homozygous for all three VE09, VE05 and VE02 loci, whereas a plant derived from their cross pollination (23 × 8) was heterozygous for the same markers.
Obtainment of fertile F1 hybrids
Although all five hybrids were vegetatively normal, only three resulted fertile and successfully completed the reproductive cycle, yielding variable amounts of F2 seeds (Table 1). Figure 4a, b shows an example of the V. ervilia parental lines used for the crosses, while Fig. 4c–e reports two of the F1 hybrids, together with their parental lines. All plants of Italian accessions showed a root crown originating several stems (Fig. 4b), whereas all Turkish accessions were single stemmed (Fig. 4a). It is noteworthy that all five hybrids were multi-stemmed as the Italian parent, irrespectively of the cross direction, indicating that it was a nuclear dominant trait not subjected to parent-specific expression (i.e. imprinting, Fig. 4e). Hybrids appeared more vigorous compared to their parents (Fig. 4c, d), probably as a consequence of heterosis, even if this effect was not detected for seed production (not shown). Flowering time was differently inherited among the fertile hybrids. In a greenhouse with 12 h daylight, plants from Turkish accessions started to bloom in the second half of April (76 days after sowing, DAS, early flowering), and completed fruit ripening and seed dehydration in late May. By contrast, plants from Italian adapted accessions started to flower in May (96 DAS, late flowering). Out of the three fertile hybrids, hybrid 10 × 23 retained the early flowering trait only, while hybrids 14 × 21 and 23 × 8 showed an intermediate blooming phenotype, because they started to flower at around 80 DAS (early flowering) and completed fruit ripening in the following month (producing 113 early seeds, Table 1). Surprisingly, a second-round flowering for hybrids 14 × 21 and 23 × 8 took place at around 94 DAS, thus resembling the late flowering trait of Italian accessions, and producing a second set of 45 late seeds after another month (Table 1 and Fig. 4f–g).
Phenotypic analysis of V. ervilia parental lines and their F1 hybrids. Five-month-old plants in pots from seeds grown in greenhouse obtained by self-fertilization of the single stem Turkish accession 14 a and the three-stem Italian accession 23 b. Seven-month-old plants in pots c from seeds obtained by self-fertilization of the Turkish accession 14 (maternal parent, at left), by self-fertilization of the Italian accession 21 (paternal parent, at right), and by 14 × 21 cross (F1 hybrid, in the centre). Seven-month-old plants d from seeds obtained by self-fertilization of the Italian accession 23 (maternal parent, at left), by self-fertilization of the Turkish accession 8 (paternal parent, at right), and by the 23 × 8 cross (F1 hybrid, in the centre). Panel e shows a detail of the root crown zone of the three plants of panel c to highlight the multi-stemmed hybrid and paternal plants. Branches of the hybrid plant of panel d are shown, in particular those bearing legumes formed after the first flow of flowering f and the apical portion of the same plant stem with the second flow of flowering observed g
F2 seed production
Traits concerned with seed production were evaluated among the F2 plants of two crosses and related to their original parental lines (Table S3). Most of the evaluated traits of the cross 14 × 21 showed mean values of the F2 plants intermediate between the two initial parents, even if they were often closer to the female parent (accession 14) of Turkish origin, from which the F2 progenies did not differ significantly. The same trend was also observed in the cross 23 × 8 with Italian origin female genotype. In most traits, the mean values of the F2 generation were intermediate but statistically closer to the female parent. We cannot affirm the presence of a maternal effect in the two crosses since the traits taken into consideration are quantitative and most likely regulated by genes placed in the nuclear genome.
Discussion
To our knowledge, this is the first time in which artificial intraspecific hybrids proved by molecular markers are reported in the genuine cleistogamous species V. ervilia. This result provides an important tool to increase the genetic variability of locally adapted landraces with important agronomic traits present in the primary gene pool of this species.
As a first step, we investigated flower development with a detailed cyto-histological analysis to determine the right time for emasculation and manual cross. We observed that male meiosis started earlier than female meiosis and that ovules were of the hemianatropous type, as it occured in other legume species, in which the ovule development started as anatropous type but ended as hemianatropous, due to a different growth in the funiculus region (Bocquet 1959; Bocquet and Bersier 1960; Bouman and Boesewinkel 1991). Moreover, we reported that the chalazal megaspore developed as functional megaspore, whereas the other three megaspores were destined to degenerate. This developmental pattern was reported in most Vicia species (Mitchell 1975), although in other legume species it was observed that the second cell from the chalazal end developed into functional megaspore (Pal 1960; Cameron and Prakash 1994). According to our results, flowers must be emasculated at the end of megagametogenesis, when the petals are three-quarters of the sepal length. As well as in lentil (Lens culinaris Medikus), a self-pollinated species with small cleistogamous flowers like those of V. ervilia (Fratini et al. 2014), manual crosses must be performed when the petals reach three-quarters of the sepal length (Wilson 1972; Malhotra et al. 1978). Therefore, in both species the suitable flower developmental stage for crossing is when the flower is still closed.
Of 98 pollinated flowers representing 26 cross combinations (one cross combination involved one genotype of Italian and one genotype of Turkish origin, Table S2), 4.1% pod set, i.e. four pods yielding five hybrid seeds (5.1% hybrid seed set) as assessed by SSR analyses were obtained. These values are lower than those reported in lentil in previous green-house studies, e.g. 11% seed set in Wilson (1972) and 17.4 pod set and 24.4% seed set in Fratini et al. (2004). In Pea another cleistogamous genus almost completely self-pollinating, researches to overcome hybridization barriers are more advanced: genes controlling cross compatibility of cultivated P. sativum with wild relatives have been identified (Bogdanova et al. 2009) and interspecific hybrids have been reported (Ochatt et al. 2004; Bogdanova et al. 2009). Selfed seeds in V. ervilia were estimated to be 90.9% in comparison to the 5% reported in lentil (Fratini et al. 2004), which could mean the difficulty for the operator to identify V. ervilia flowers at the correct physiological stage of three-quarters sepals to petals to prevent self-fertilization. Alternatively, we could have got a high rate pollen contamination during emasculation due to anther rupture and pollen release inside the flower. Even without investigating the influence of genotype on crossing efficiency, we noted that Italian accession 21 resulted the worst as concerns megasporogenesis/megagametogenesis development (with the highest percentage of both degenerated megaspore and ovule abortion) and microgametogenesis (with the highest percentage of sterile pollen). Actually, no hybrid was obtained in cross combinations where accession 21 was used as female parent, whereas one cross combination with accession 21 as male parent produced two hybrids, therefore the relatively high percentage of pollen abnormalities did not affect the obtainment of hybrid progenies in crossing procedures.
All crosses were performed manually under controlled environmental conditions, as reported in lentil by Fratini et al. (2004) who compared the crossing efficiency both in the greenhouse and in the field. SSR analysis confirmed the higher level of intra-population variability already detected for Turkish landraces (Russi et al. 2019) compared to Italian landraces as several genotypes were identified within the two accessions 10 and 14. In any case, we successfully obtained inter-landrace hybrids that showed heterozygosity for all the markers used. Moreover, the hybrids generated F2 plants that showed various arrays of seed production and growth habit, at least under greenhouse growing conditions. Phenotype evaluation of the F3 generation in field trials will provide definitive evidence of obtaining superior genotypes of V. ervilia for both seed production and growth habit. Our findings lay the basis for a hybridization-based route for the genetic improvement of bitter vetch in the perspective to exploit this versatile crop under low input farming system.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Akaffou DS, Konate I, Sié RS, Poncet V, Bi IAZ, Keli J, Legnate H, Kochko HA, Hamon S, Hamon P (2014) Flowering phenology and yield-related traits in an interspecific cross between Coffea pseudozanguebariae Bridson and C. canephora Pierre. Austr J Crop Sci 8:1272–1280
Ates S, Feindel D, El-Moneim AM, Ryan J (2014) Annual forage legumes in dryland agricultural systems of the West Asia and North Africa Regions: research achievements and future perspective. Grass Forage Sci 69:17–31. https://doi.org/10.1111/gfs.12074
Berger JD, Robertson LD, Cocks PS (2002) Agricultural potential of Mediterranean grain and forage legumes: key differences between and within Vicia species in terms of phenology, yield, and agronomy give insight into plant adaptation to semi-arid environments. Genet Resour Crop Ev 49:313–325
Bocquet G (1959) The campylotropous ovule. Phytomorphology 9:222–227
Bocquet G, Bersier JD (1960) La valeur systématique de l’ovule: développements tératologiques. Arc Sci 13:475–496
Bogdanova VS, Galieva ER, Kosterin OE (2009) Genetic analysis of nuclear–cytoplasmic incompatibility in pea associated with cytoplasm of an accession of wild subspecies Pisum sativum subsp. elatius (Bieb.) Schmahl. Theor Appl Genet 118:801–809. https://doi.org/10.1007/s00122-008-0940-y
Bouman F, Boesewinkel FD (1991) The campylotropous ovules and seeds, their structure and functions. Bot Jahrbücher Für Systematik 113:255–270
Cameron B, Prakash N (1994) Variations of the megagametophyte in the Papilionoideae. In: Ferguson IK, Tucker S (eds) Advances in legume systematics. Kew: Royal Botanical Gardens, England, pp 97–115
Christie K, Strauss SY (2019) Reproductive isolation and the maintenance of species boundaries in two serpentine endemic Jewelflowers. Evolution 73:1375–1391. https://doi.org/10.1111/evo.13767
Culley TM, Klooster MR (2007) The cleistogamous breeding system: a review of its frequency, evolution, and ecology in angiosperms. Bot Rev 73:1–30
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2012) InfoStat versión 2012. InfoStat Group, Facultad de Ciencias Agropecuarias, Universidad Nacioal de Córdoba, Argentina. URL http://www.infostat.com.ar
El Fatehi S, Béna G, Sbabou L, Filali-Maltouf A, Ater M (2013) Preliminary results for use SSR markers in bitter vetch “Vicia ervilia (L.) Willd”. Int J Res Agric and Food Sci 1:40–46
Esteban J (1996). El Yero [Bitter vetch]. In: Franco Jubete F, Ramos Monreal A (eds) El cultivo de las Leguminosas de grano en Castilla y Leon. Junta de Castilla y León. Consejería de Cultura y Turismo, pp. 161–193
Fratini R, Ruiz ML, Pérez de la Vega M (2004) Intraspecific and inter-subspecific crossing in lentil (Lens culinaris Medik.). Can J Plant Sci 84:981–986. https://doi.org/10.4141/P03-201
Fratini RM, Pérez de la Vega M, Ruiz Sánchez ML (2014) Lentil. In: Singh M, Bisht IS, Dutta M (eds) Broadening the genetic base of grain legumes. Springer, New Delhi, pp 75–93. https://doi.org/10.1007/978-81-322-2023-7_6
Hadley HH, Openshaw SJ (1980) Interspecific and intergenetic hybridization. In: Feher WR, Hadley HH (eds) Hybridisation of crop plants. The America Society of Agronomy and the Crop Science Society of America Publisher, Madison, pp 105–257
Huang D, Gao L, McAdams J, Zhao F, Lu H, Wu Y, Martin J, Sherif MS, Subramanian J, Duan H, Liu W (2023) Engineered cleistogamy in Camelina sativa for bioconfinement. Hortic Res 10:uhac280. https://doi.org/10.1093/hr/uhac280
Koontz SM, Weekley CW, Haller Crate SJ, Menges ES (2017) Patterns of chasmogamy and cleistogamy, a mixed-mating strategy in an endangered perennial. AoB Plants 9:plx059. https://doi.org/10.1093/aobpla/plx059
Ladizinsky G (1998) Plant evolution under domestication. Kluwer Academic Publishers, Dordrecht. https://doi.org/10.1007/978-94-011-4492-z
Ladizinsky G, van Oss H (1984) Genetic relationships between wild and cultivated Vicia ervilia (L.) Willd. Bot J Linn Soc 89:97–100. https://doi.org/10.1111/j.1095-8339.1984.tb01003.x
Lande R, Schemske DW (1985) The evolution of self-fertilization and inbreeding depression in plants. I Genetic Models Evolution 39:24–40. https://doi.org/10.1111/j.1558-5646.1985.tb04077.x
Livanios I, Lazaridi E, Bebeli PJ (2018) Assessment of phenotypic diversity in bitter vetch (Vicia ervilia (L.) Willd.) populations. Genet Resour Crop Evol 65:355–371. https://doi.org/10.1007/s10722-017-0539-8
Lloyd DG (1979) Some reproductive factors affecting the selection of self-fertilization in plants. Am Nat 113:67–79. https://doi.org/10.1086/283365
Lord EM (1981) Cleistogamy: a tool for the study of floral morphogenesis, function and evolution. Bot Rev 47:421–442
Malhotra RS, Balyan HS, Gupta PK (1978) Crossing Technique in Lentils. Lens Newslet 5:7–8
Mitchell JP (1975) Megasporogenesis and microsporogenesis in Vicia faba. Can J Bot 23:2804–2812. https://doi.org/10.1139/b75-308
Ochatt SJ, Benabdelmouna A, Marget P, Aubert G, Moussy F, Pontécaille C, Jacas L (2004) Overcoming hybridization barriers between pea and some of its wild relatives. Euphytica 137:353–359. https://doi.org/10.1023/B:EUPH.0000040476.57938.81
Ordóñez-Fernández R, Repullo-Ruibérriz de Torres MA, Márquez-García J, Moreno-García M, Carbonell-Bojollo RM (2018) Legumes used as cover crops to reduce fertilisation problems improving soil nitrate in an organic orchard. Eur J Agron 95:1–13. https://doi.org/10.1016/j.eja.2018.02.001
Pal N (1960) Development of the seed of Milletia ovalifolia. Bot Gaz 122:130–137. https://doi.org/10.1086/336098
Porta R, Di Pierro P, Roviello V, Sabbah M (2017) Tuning the functional properties of bitter vetch (Vicia ervilia) protein films grafted with spermidine. Int J Mol Sci 18:2658–2669. https://doi.org/10.3390/ijms18122658
Ratinam M, El-Moneim AM, Saxena MC (1994) Variation in sugar content and dry matter distribution in roots and their associations with frost tolerance in certain forage legume species. J Agron Crop Sci 173:345–353. https://doi.org/10.1111/j.1439-037X.1994.tb00582.x
Russi L, Acuti G, Trabalza-Marinucci M, Porta R, Rubini A, Damiani F, Cristiani S, Dal Bosco A, Martuscelli G, Bellucci M, Pupilli F (2019) Genetic characterisation and agronomic and nutritional value of bitter vetch (Vicia ervilia), an under-utilised species suitable for low-input farming systems. Crop Pasture Sci 70:606–614. https://doi.org/10.1071/CP19079
SAS Institute. SAS OnDemand for Academics. Release 3.81 SAS Institute Inc., Cary, NC, USA.
Schoen DJ, Lloyd DG (1984) The selection of cleistogamy and heteromorphic diaspores. Biol J Linn Soc 23:303–322. https://doi.org/10.1111/j.1095-8312.1984.tb00147.x
Sun J, Mooney H, Wu W, Tang H, Tong Y, Xu Z, Huang B, Cheng Y, Yang X, Wei D, Zhang F, Jianguo L (2018) Importing food damages domestic environment: evidence from global soybean trade. P Natl Acad Sci USA 115:5415–5419. https://doi.org/10.1073/pnas.1718153115
Verstringe S, Vandercruyssen R, Carmans H, Rusu AV, Bruggeman G, Trif M (2023) Alternative Proteins for Food and Feed. In: Galanakis CM (ed) Biodiversity, Functional Ecosystems and Sustainable Food Production. Springer, Cham. https://doi.org/10.1007/978-3-031-07434-9_10
Wilson VE (1972) Morphology and technique for crossing Lens esculenta Moench. Crop Sci 12:231–232. https://doi.org/10.2135/cropsci1972.0011183X001200020026x
Winn AA, Moriuchi KS (2009) The maintenance of mixed mating by cleistogamy in the perennial violet Viola septemloba (Violaceae). Am J Bot 96:2074–2079. https://doi.org/10.3732/ajb.0900048
Zhang X, Mosjidis JA (1995) Breeding systems of several Vicia species. Crop Sci 35:1200–1202. https://doi.org/10.2135/cropsci1995.0011183X003500040049x
Acknowledgements
This work was supported by the Ministero dell’agricoltura, della sovranità alimentare e delle foreste of Italy (Project VALEABO, prot.9385, date Dec 17th 2020). Thanks are due to Dr. Luca Scutigliani for his help.
Funding
Open access funding provided by Consiglio Nazionale Delle Ricerche (CNR) within the CRUI-CARE Agreement. This work was supported by the Ministero dell’agricoltura, della sovranità alimentare e delle foreste of Italy (Project VALEABO, prot.9385, date Dec 17th 2020).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Maria Eugenia Caceres, Andrea Rubini, Francesca de Marchis, Luigi Russi and Marilena Ceccarelli. The first draft of the manuscript was written by Fulvio Pupilli and Michele Bellucci and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caceres, M.E., Rubini, A., Russi, L. et al. Obtainment of intraspecific hybrids in strictly cleistogamous Vicia ervilia (L.) Willd.. Euphytica 220, 111 (2024). https://doi.org/10.1007/s10681-024-03371-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-024-03371-w