Abstract
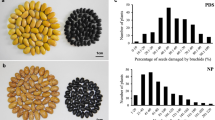
The damage caused to stored seed by bruchids (Callosobruchus maculatus) is considered to be a major production constraint in rice bean (Vigna umbellata). Breeding for genetically determined resistance is the most environmentally benign and cost-effective means to mitigate the losses to bruchid infestation. Here, a screen of rice bean germplasm identified two sources of resistance, and determined the genetic basis of the resistance using a quantitative trait locus (QTL) mapping approach. The two resistant accessions (LRB238 and JP100304) were each crossed to a common susceptible cultivar (LRB26) to generate F2 mapping populations, one of which (LRB238 × LRB26) was genotyped with a range of Vigna sp. microsatellite assays and by sequence related amplified polymorphism (SRAP) fingerprinting. The resulting linkage map comprised ten linkage groups and covered a genetic distance of 872.1 cM with a mean inter-marker distance of 32.05 cM. The subsequent QTL analysis detected the presence of 11 QTL, distributed over all ten linkage groups, most of which were associated with the % damage caused to the seed. Two major QTL, Cmpd1.5 (flanked by the SRAP markers E2M9-270 and E12M7-311) and Cmpd1.6 (flanked by the SRAP marker E7M10-141 and the microsatellite locus CEDG259) mapped within 11.9 cM and 13.0 cM of the flanking markers, respectively, accounted for, 67.3 and 77.4 % of the variance respectively, for % damaged seeds. A bulked segregation analysis carried out in the JP100304 × LRB26 population revealed that the resistance donor harboured some resistance factors not represented in LRB238.



Similar content being viewed by others
References
Adjadi O, Singh BB, Singh SR (1985) Inheritance of bruchid resis- tance in cowpea. Crop Sci 25:740–742
Appleby JH, Credland PF (2003) Variation in responses to susceptible and resistant cowpeas among WestAfrican populations of Callosobruchus maculatus (Coleoptera:bruchidae). J Econ Entomol 96:489–502
Baouaa IB, Margamb V, Amadoua L, Murdock LL (2012) Performance of triple bagging hermetic technology for postharvest storage of cowpea grain in Niger. J Stored Prod Res 51:81–85
Blair MW, Pedraza F, Buendia HF, Gaitan-Soli SE, Beebe SE, Gepts P, Thome J (2003) Development of a genome-wide anchored microsatellite map of common bean (Phaseolus vulgaris L.). Theor Appl Genet 107:1362–1374
Budak H, Shearman RC, Parmaksiz I, Gaussoin RE, Riosdan TP, Dweikat I (2004) Molecular characterization of Buffalograss germplasm using sequence-related amplified polymorphism markers. Theor Appl Genet 108:328–334
Buso GSC, Amaral ZPS, Brondani RPV, Ferreira ME (2006) Microsatellite markers for the common bean (Phaseolus vulgaris). Mol Ecol Notes 6:252–254
Carvalho NM de, Vieria RD (1996). Ricebean [Vigna umbellata (Thunb.) Ohwi and Ohashi]. In: Nkowolo E, Smartt J (eds). Legumes and oilseeds in nutrition. Chapman and Hall, pp 222–228
Chaitieng B, Kaga A, Tomooka N, Isemura T, Kuroda Y, Vaughan DA (2006) Development of a black gram [Vigna mungo (L.) Hepper] linkage map and its comparison with an azuki bean [Vigna angularis (Willd.) Ohwi and Ohashi] linkage map. Theor Appl Genet 113:1261–1269
Cheng XZ, Wang SH, Wu SY, Zhou JH, Wang SM, Charles YY (2005) Tagging and utilization bruchid resistance gene using PCR markers in mung bean. Agric Sci China 4:579–583
Choi HK, Mun JH, Kim DJ, Zhu H, Baek JM, Mudge J, Roe B, Ellis N, Doyle J, Kiss GB, Young ND, Cook DR (2004) Estimating genome conservation between crop and model legume species. Proc Natl Acad Sci USA 101:15389–15394
Choumane W, Winter P, Weigand F, Kahl G (2004) Conservation of microsatellite flanking sequences in different taxa of leguminosae. Euphytica 138:239–245
Datta S, Kaashyap M, Kumar S (2010a) Amplification of chickpea-specific SSR primers in Cajanus species and their validity in diversity analysis. Plant Breed 129:334–340
Datta S, Mahfooz S, Singh P, Choudhary AK, Singh F, Kumar S (2010b) Cross-genera amplification of informative microsatellite markers from common bean and lentil for the assessment of genetic diversity in pigeonpea. Physiol Mol Biol Plants 16:123–134
Dua RP, Raiger HL, Phogat BS, Sharma SK (2009). Underutilized crops: Improved varieties and cultivation practices. All India Coordinated Research Network (Underutilized crops), National Bureau of Plant Genetic Resources (NBPGR) publications, New Delhi. pp. 21–25
Espósito MA, Martin EA, Cravero VP, Cointry E (2007) Characterization of pea accessions by SRAP’s markers. Sci Hortic 113(4):329–335
Fernandez GCJ, Talekar NS (1990) In: Fujii K, Gatehouse AMR, Johnson D, Mitchel R, Yoshida T (eds) Bruchids and Legumes: Economics. Ecology and Coevolution, Kluwer Academic Publishers, Dordrecht, pp 209–217
Ferriol MB, Pico P, de Córdova F, Nuez F (2004) Molecular diversity of a germplasm collection of squash (Cucurbita moschata) determined by SRAP and AFLP markers. Crop Sci 44:653–664
Fujii K, Ishimoto M, Kitamura K (1989) Patterns of resistance to bean weevils (Bruchidae) in Vigna radiata-mungo-sublobata complex inform the breeding of new resistant varieties. Appl Entomol Zool 24:126–132
Gaitan-Solis E, Duque MC, Edwards KJ, Thome J (2002) Microsatellite repeats in common bean (Phaseolus vulgaris): isolation, characterization and cross-species amplification in Phaseolus ssp. Crop Sci 42:2128–2136
Guerra-Sanz JM (2004) New SSR markers of Phaseolus vulgaris from sequence databases. Plant Breeding 123:87–89
Guohao H, Woullard FE, Marong I, Guo BZ (2006) Transferability of soybean SSR markers in peanut (Arachis hypogaea L.). Peanut Sci 33:22–28
Gupta SK, Gopalakrishna T (2009) Genetic diversity analysis in blackgram (Vigna mungo (L.) Hepper) using AFLP and transferable microsatellite markers from azuki bean (Vigna angularis (Willd.) Ohwi & Ohashi). Genome 52:120–129
Gwag JG, Chung WK, Chung HK, Lee JH, Ma KH, Dixit A, Park YJ, Cho EG, Kim TS, Lee SH (2006) Characterization of new microsatellite markers in mung bean, Vigna radiata (L.). Mol Ecol Notes 6:1132–1134
Han OK, Kaga A, Isemena T, Wang XW, Tomooka N, Vaughan DA (2005) A genetic linkage map for azuki bean [Vignaangularis (Willd) Ohwi and Ohashi]. Theor Appl Genet 111:1278–1287
Isemura T, Kaga A, Konishi S, Ando T, Tomooka N, Han OK, Vaughan DA (2007) Genome dissection of traits related to domestication in azuki bean (Vigna angularis) and comparison with other warm-season legumes. Ann Bot 100:1053–1071
IsemuraT Kaga A, Tomooka N, Shimizu T, Vaughan DA (2010) The genetics of domestication of rice bean, Vigna umbellata. Ann Bot 106:927–944
Kaga A, Isemura T, Tomooka N, Vaughan DA (2008) The GenetiCs of domestication of the Azuki Bean (Vigna angularis). Genetics 178(2):1013–1036
Kaur D, Kapoor AC (1992) Nutrient composition and antinutritional factors of ricebean. Food Chem 43:119–124
Kearsey MJ, Pooni HS (1996) The genetical analysis of quantitative traits. Chapman and Hall, London, pp 138–139
Khadka K, Acharya BD (2009) Cultivation practices of ricebean. Research and Development (LI-BIRD), Local Initiatives for Biodiversity, Pokhara
Kitamura K, Ishimoto M, Sawa M (1988) Inheritance of resistance to infestation with azuki bean weevil in Vigna sublobata and successful incorporation to V. radiata. Jpn J Breed 38:459–464
Kongjaimun A, Kaga A, Tomooka N, Somta P, Shimizu T, Shu Y, Vaughan DA, Srinives P (2012) An SSR-based linkage map of yardlong bean [Vigna unguiculata ssp. sesquipedalis (L.) Verc.] and QTL analysis of pod length. Genome 55:81–92
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Kumar SV, Tan G, Quah SC, Yusoff K (2002) Isolation of microsatellite markers in mung bean, Vigna radiata. Mol Ecol Notes 2:96–98
Lawn RJ (1995) The Asiatic Vigna species. In: Smartt J, Simmonds NW (eds) Evolution of Crop Plants. Longman Scientific and Technical, Harlow, pp 321–326
Li CD, Fatokun CA, Ubi B, Singh BB, Scoles GJ (2001) Determining genetic similarities and relationships among cowpea breeding lines and cultivars by microsatellite markers. Crop Sci 41:189–197
Li CF, Quiros G (2001) Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in Brassica. Theor Appl Genet 103:455–461
Lodhi MA, Ye GN, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Rep 12:6–13
Menancio-Hautea DI, Kumar L, Danesh ND (1993) A genome map for mung bean [Vigna radiate (L.) Wilczek] based on DNA genetic markers (2n = 2x = 22). In Genetic maps 1992. A compilation of linkage and restriction maps of genetically studied organisms. Cold Spring Harbor Laboratory Press, New York. pp 259–261
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci 88:9828–9832
Murdock LL, Seckb D, Ntoukamc G, Kitchd L, Shadea RE (2003) Preservation of cowpea grain in sub-Saharan Africa-Bean/Cowpea CRSP contributions. Field Crops Res 82:169–178
Myrna S, Rodriguez Evelyn Mae T, Mendoza EMT (1991) Nutritional assessment of seed proteins in rice bean [Vigna umbellata (Thumb.) Ohwi and Ohashi]. Plant Foods Hum Nutr 41:1–9
Ooijen VJW, Voorrips RE (2001) JoinMap-3.0: software for the calculation of genetic linkage maps. Plant Research International, Wageningen
Peakall R, Gilmore S, Keys W, Morgante M, Rafalski A (1998) Cross-species amplification of soybean (Glycine max) SSRs within the genus and other legume genera: implication for the transferability of SSRs in plants. Mol Biol Evol 15:1275–1287
Seehalak W, Somta P, Musch W, Srinives P (2009) Microsatellite markers for mung bean developed from sequence database. Mol Ecol Resour 9:862–864
Snedecor GW, Cochran WG (1994) Statistical methods, 8th edn. Iowa state University Press, Ames
Somta P, Musch W, Kongsamai B, Chanprame S, Nakasathien S, Toojinda T, Sorajjapinun W, Seehaluk W, Tragoonrung S, Srinives P (2008a) New microsatellite markers isolated from mung bean (Vigna radiata (L.) Wilczek). Mol Ecol Resour 8:1155–1157
Somta P, Ammaranan C, Ooi PAC, Srinives P (2007) Inheritance of seed resistance to bruchids in cultivated mung bean (Vigna radiata L. Wilczek). Euphytica 155:47–55
Somta P, Kaga A, Tomooka N, Isemura T, Vaughan DA, Srinives P (2008b) Mapping of quantitative trait loci for a new source of resistance to bruchids in the wild species Vigna nepalensis Tateishi and Maxted (Vigna subgenus Ceratotropis). Theor Appl Genet 117:621–627
Somta P, Kaga A, Tomooka N, Kashiwaba K, Isemura K, Chaitieng B, Srinives P, Vaughan DA (2006) Development of an interspecific Vigna linkage map between Vigna umbellata (Thunb.) Ohwi & Ohashi and V. Nakashimae (Ohwi) Ohwi & Ohashi and its use in analysis of bruchid resistance and comparative genomics. Plant Breeding 125:77–84
Somta P, Seehalak W, Srinives P (2009) Development, characterization and cross-species amplification of mung bean (Vignaradiata) genic microsatellite markers. Conserv Genet 10:1939–1943
Souframanien J, Gupta SK, Gopalakrishna T (2010) Identification of quantitative trait loci for bruchid (Callosobruchus maculatus) resistance in black gram [Vigna mungo (L.) Hepper]. Euphytica 176:349–356
Southgate BJ (1979) Biology of bruchidae. Ann Rev Entomol 24:449–473
Tangphatsornruang S, Somta P, Uthaipaisanwong P, Chanprasert J, Sangsrakru D, Seehalak W, Sommanas W, Tragoonrung S, Srinives P (2009) Characterization of microsatellites and gene contents from genome shotgun sequences of mung bean (Vignaradiata (L.) Wilczek). BMC Plant Biol 9:137–145
Tomooka N (2009) The origin of rice bean (Vigna umbellata) and azuki bean (V. angularis): the evolution of two lesser-known Asian beans. In: Akimiti T (ed) An illustrated eco-history of the Mekong River Basin. Bangkok: White Lotus Co
Tomooka N, Vaughan DA, Moss H, Maxted N (2002) The Asian Vigna: genus Vigna subgenus Ceratotropis genetic resources. Kluwer Academic Publishers, Dordrecht
Vavilov NI (1951) Origin, variation, immunity and breeding of cultivated plants. Chronic Bot 13:1–1364
Vos P, Hogers R, Bleeker M, Reijans M, Van De Lee T, Hornes M, Frijters A, Pot J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wang LX, Cheng XZ, Wang SH, Liu CY (2009) Transferability of SSR markers for adzukibean into mung bean. Acta Agron Sin 35:816–820
Wang XW, Kaga A, Tomooka N, Vaughan DA (2004) The development of SSR markers by a new method in plants and their application to gene flow studies in azuki bean [Vigna angularis (Willd.) Ohwi and Ohashi]. Theor Appl Genet 109:352–360
Young ND, Kumar L, Menancio-Hautea D, Danesh D, Talekar NS, Shanmugasundarum S, Kim DH (1992) RFLP mapping of a major bruchid resistance gene in mung bean. Theor Appl Genet 84:839–844
Zevan AC, Zhukovsky PM (1975) Dictionary of cultivated plants and their centres of diversity. Centre for Agriculture publishing and documentation, Wageningen
Acknowledgments
The authors are grateful to the Kirkhouse Trust (Oxford, UK) for funding the senior author’s Ph. D. programme (Grant number KSGIN00135). The constructive advice given by Dr. Robert Koebner (Norwich, UK) is particularly acknowledged. We also thank Dr. Prashanth Mohan Raj, National Bureau of Agriculturally Important Insects for providing the C. m aculatus culture, and NIAS (Japan) for the gift of accession JP100304.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkataramana, P.B., Gowda, R., Somta, P. et al. Mapping QTL for bruchid resistance in rice bean (Vigna umbellata). Euphytica 207, 135–147 (2016). https://doi.org/10.1007/s10681-015-1551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1551-8