Abstract
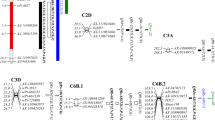
A well organized root system is of great importance in plants for better anchorage and efficient nutrient use. Two wheat populations were used to map QTLs associated with root traits. A double haploid population contains 216 lines and derived from a cross between Nongda 3338 and Jingdong 6. The RIL progeny includes 217 lines which were evolved from another cross between Nongda 3331 and Zang 1817. Root morphological parameters were measured in seedling stage using hydroponic culture technique. Total root length, root surface area, root volume, number of root tips and main root length were measured for both the populations. In total, 54 QTLs for root traits were detected. Among the QTLs detected, 39 QTLs distributed on chromosomes 2A, 2B, 3A, 4B, 4D, 5A, 6A, 6D, and 7B were identified in DH population, while 15 QTLs on chromosomes 1B, 2B, 3B, 4A, 4D, 5A, 5B and 7A were identified in the RIL population. QTLs were clustered in 8 genomic regions in DH and 4 genomic regions in RIL population. Important QTL rich regions on chromosome 2A (wsnp_Ex_c19516_28480622-Xgwm614b), 3A (Excalibur_c24354_465-Kukri_rep_c102151_697 and Xwmc695-IAAV5821) and 4D (RAC875_c5827_554-wsnp_BF473052D_Ta_2_1) in DH and 3B (Xbarc115-Xwmc291), 4A (Xcwem34-Xbarc28b) and 4D (Xbarc1118-Rht2) in RIL population were found as they had pleiotropic effect for controlling root traits. Negative correlation was found between root traits and plant height in both populations. Root traits was found unaffected by Rht2 gene. Major QTLs detected on chromosome 4D for root traits might be different from the QTL detected previously for plant height.






Similar content being viewed by others
References
An DG, Su JY, Liu QY, Zhu YG, Tong YP, Li JM, Jing RL, Li B, Li ZS (2006) Mapping QTLs for nitrogen uptake in relation to the early growth of wheat (Triticum aestivum L.). Plant Soil 284:73–84
Bai C, Liang Y, Hawkesford MJ (2013) Identification of QTL associated with seedling root traits and their correlation with plant height in wheat. J Exp Botany 64(6):1745–1753
Basten C, Weir BS, Zeng ZB (1994) Zmap—a QTL cartographer. In: Smith C, Gavora JS, Benkel B, Chesnais J, Fairfull W, Gibson JP, Kennedy BW, Burnside EB (eds) Proceedings of the 5th world congress on genetics applied to livestock production: computing strategies and software, Organizing Committee, Guelph vol 22, pp 85–66
Börner A, Schumann E, Fürste A, Cöster H et al (2002) Mapping of quantitative trait loci determining agronomic important characters in hexaploid wheat (Triticum aestivum L.). Theor Appl Genet 105:921–936
Bush MG, Evans LT (1988) Growth and development in tall and dwarf isogenic lines of spring wheat. Field Crops Res 18:243–270
Churchill G, Doerge R (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK (2005) An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica 142:169–196. doi:10.1007/s10681-005-1681-5
Cui F, Li J, Ding AM, Zhao CH, Wang L, Wang X, Li S, Bao Y, Li X, Feng D, Kong L, Wang H (2011) Conditional QTL mapping for plant height with respect to the length of the spike and internode in two mapping populations of wheat. Theor Appl Genet 122:1517–1536
Fitter AH (2002) Characteristics and functions of root systems. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. Marcel Dekker, New York, pp 249–259
Gregory PJ (2006) Genetic control of root system properties. Plant roots: growth, activity and interactions with soils. Blackwell, Oxford, pp 253–282
Herder GD, Isterdael GV, Beeckman T, Smet ID (2010) The roots of a new green revolution interactions for grain yield. Trends Plant Sci 15:600–607
Ibrahim SE, Schubert A, Pillen K, Léon J (2012) QTL analysis of drought tolerance for seedling root morphological traits in an advanced backcross population of spring wheat. Int J AgriSci 2(7):619–629
Kimurto PK, Kinyua MG, Birech R, Korir PC, Njoka EM, Njau PN (2005) Root and shoot characteristics as selection criteria for drought tolerance in bread wheat (Triticum aestivum L.) at seedling stage under tropical environment. Discov Innov 17:74–84
Li Z, Ni Z, Peng H, Liu Z, Nie X, Xu L, Liu G, Sun X (2007) Molecular mapping of QTLs for root response to phosphorus deficiency at seedling stage in wheat (Triticum aestivus L.). Prog Nat Sci 17(10):1177–1184
Liu X, Li R, Chang X, Jing R (2013) Mapping QTLs for seedling root traits in a doubled haploid wheat population under different water regimes. Euphytica 189:51–66
Liu G, Jia L, Lu L, Qin D, Zhang J, Guan P, Ni Z, Yao Y, Sun Q, Peng H (2014) Mapping QTLs of yield-related traits using RIL population derived from common wheat and Tibetan semi-wild wheat. Theor Appl Genet 127:2415–2432
Lynch JP (2007) Roots of the second green revolution. Aust J Bot 55:493–512
Manschadi AM, Hammer GL, Christopher JT, deVoil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129
McKey J (1973) The wheat root. In: Proceedings 4th international wheat genetics symposium. Missouri Agricultural Experiment Station, College of Agriculture, University of Missouri, Columbia, Missouri, USA, pp 827–842
McPhee K (2005) Variation for seedling root architecture in the core collection of pea germplasm. Crop Sci 45:1758–1763
Miralles DJ, Slafer GA, Lynch V (1997) Rooting patterns in near-isogenic lines of spring wheat for dwarfism. Plant Soil 197:79–86
Narayanan S, Mohan A, Gill KS, Prasad PVV (2014) Variability of root traits in spring wheat germplasm. PLoS One 9(6):e100317. doi:10.1371/journal.pone.0100317
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607
Rahnama A, Munns R, Poustini Watt M (2011) A screening method to identify genetic variation in root growth response to a salinity gradient. J Exp Bot 62(1):69–77
Ren Y, He X, Liu D, Li J, Zhao X, Li B, Tong Y, Zhang A, Li Z (2012) Major quantitative trait loci for seminal root morphology of wheat seedlings. Mol Breed 30:139–148
Salvi S, Tuberosa R (2005) To clone or not to clone plant QTLs: present and future challenges. Trends Plant Sci 10:297–304
Sanguineti MC, Li S, Maccaferri M, Corneti S, Rotondo S, Chiari T, Tuberosa R (2007) Genetic dissection of seminal root architecture in elite durum wheat germplasm. Ann Appl Biol 151:291–305
Serraj R, Krishnamurthy L, Kashiwagi J, Kumar J, Chandra S, Crouch JH (2004) Variation in root traits of chickpea (Cicer arietinum L.) grown under terminal drought. Field Crops Res 88:115–127
Sharma S, Xu S, Ehdaie B, Hoops A, Close TJ, Lukaszewski AJ, Waines JG (2011) Dissection of QTL effects for root traits using a chromosome arm-specific mapping population in bread wheat. Theor Appl Genet 122:759–769
Siddique HM, Belford RK, Tennant D (1990) Root: shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant Soil 121:89–98
Su JY, Zheng Q, Li HW, Li B, Jing RL, Tong YP, Li ZS (2009) Detection of QTLs for phosphorus use efficiency in rela-tion to agronomic performance of wheat grown under phosphorus sufficient and limited conditions. Plant Sci 176:824–836
Subbiah BV, Katyal JC, Narasimh RL, Dakshina C (1968) Preliminary investigations on root distribution of high yielding wheat varieties. Int J Appl Radiat Isot 19:385–390
Tuberosa R, Sabguineti MC, Landi P, Giuliani MM, Salvi S, Conti S (2002) Identification of QTLs for root character-istics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Mol Breed 48:697–712
Wojciechowski T, Gooding MJ, Ramsay L, Gregory PJ (2009) The effects of dwarfing genes on seedling root growth of wheat. J Exp Bot 60:2565–2573
Yano M (2001) Genetic and molecular dissection of naturally occurring variations. Curr Opin Plant Biol 4:130–135
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Zhou X, Jing R, Hao Z, Chang X, Zhang Z (2005) Mapping QTL for seedling root traits in common wheat. Scientia Agricultura Sinica 38(10):1951–1957
Zhu J, Kaeppler SM, Lynch JP (2005) Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor Appl Genet 111:688–695
Acknowledgments
This work was financially supported by 863 Project of China (2012AA10A309).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kabir, M.R., Liu, G., Guan, P. et al. Mapping QTLs associated with root traits using two different populations in wheat (Triticum aestivum L.). Euphytica 206, 175–190 (2015). https://doi.org/10.1007/s10681-015-1495-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1495-z