Abstract
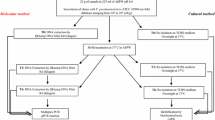
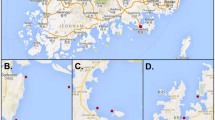
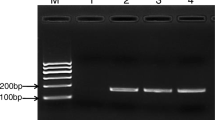
Oysters can accumulate potentially pathogenic water bacteria. The objective of this study was to compare two procedures to quantify Vibrio species present in oysters to determine the most sensitive method. We analyzed oyster samples from the Gulf of Mexico, commercialized in Mexico City. The samples were inoculated in tubes with alkaline peptone water (APW), based on three tubes and four dilutions (10−1 to 10−4). From these tubes, the first quantification of Vibrio species was performed (most probable number (MPN) from tubes) and bacteria were inoculated by streaking on thiosulfate-citrate-bile salts-sucrose (TCBS) petri dishes. Colonies were isolated for a second quantification (MPN from dishes). Polymerase chain reaction (PCR) was used to determine species with specific primers: ompW for Vibrio cholerae, tlh for Vibrio parahaemolyticus, and VvhA for Vibrio vulnificus. Simultaneously, the sanitary quality of oysters was determined. The quantification of V. parahaemolyticus was significantly higher in APW tubes than in TCBS dishes. Regarding V. vulnificus counts, the differences among both approaches were not significant. In contrast, the MPNs of V. cholerae obtained from dishes were higher than from tubes. The quantification of MPNs through PCR of V. parahaemolyticus and V. vulnificus obtained from APW was sensitive and recommendable for the detection of both species. In contrast, to quantify V. cholerae, it was necessary to isolate colonies on TCBS prior PCR. Culturing in APW at 42 °C could be an alternative to avoid colony isolation. The MPNs of V. cholerae from dishes was associated with the bad sanitary quality of the samples.

Similar content being viewed by others
References
Alam, M. J., Tomochika, K. I., Miyoshi, S. I., & Shinoda, S. (2002). Environmental investigation of potentially pathogenic Vibrio parahaemolyticus in the Seto-Inland Sea, Japan. FEMS Microbiology Letters, 208(1), 83–87.
APHA, AWWA, WEF. (2012). Standard methods for the examination of water and wastewater. 22th edition. Washington, DC: American Public Health Association.
Barrera-Escorcia, G., & Wong-Chang, I. (2014). Diagnóstico y tendencias de la contaminación microbiológica en el golfo de México. In A. V. Botello, J. Rendón von Osten, J. Benítez, & G. Gold-Boucht (Eds.), Golfo de México, contaminación e impacto ambiental: diagnóstico y tendencias (pp. 595–619). Mérida: UAC, UNAM-ICMYL, CINVESTAV.
Blanco-Abad, V., Ansede-Bermejo, J., Rodriguez-Castro, A., & Martinez-Urtaza, J. (2009). Evaluation of different procedures for the optimized detection of Vibrio parahaemolyticus in mussels and environmental samples. International Journal of Food Microbiology, 129(3), 229–236.
Cabanillas-Beltrán, H., Llausás-Magaña, E., Romero, R., Espinoza, A., García-Gasca, A., Nishibuchi, M., Ishibashi, M., & Gómez-Gil, B. (2006). Outbreak of gastroenteritis caused by the pandemic Vibrio parahaemolyticus O3:K6 in Mexico. FEMS Microbiology Letters, 265(1), 76–80.
DOF: Diario Oficial de la Federación (2011). Norma Oficial Mexicana NOM-242-SSA1-2009, Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba. México: Diario Oficial de la Federación, 10 de febrero. (Avaiable at: http://dof.gob.mx/nota_detalle_popup.php?codigo=5177531).
Depaola, A., Kaysner, C. A., & Mcphearson, R. M. (1987). Elevated temperature method for recovery of Vibrio cholerae from oysters (Crassostrea gigas). Applied and Environmental Microbiology, 53(5), 1181–1182.
Depaola, A., Jones, J. L., Woods, J., Burkhardt, W., III, Calci, K. R., Krantz, J. A., Bowers, J. C., Kasturi, K., Byars, R. H., Jacobs, E., Williams-Hill, D., & Nabe, K. (2010). Bacterial and viral pathogens in live oysters: 2007 United States market survey. Applied and Environmental Microbiology, 76(9), 2754–2768.
Hara-Kudo, Y., Miwa, N., Yamasaki, S., Yatsuyanagi, J., Iwade, Y., Takahash, H., & Miyasaka, J. (2005). Quantitative detection of Vibrio vulnificus in seafood. Kansenshogaku Zasshi, 79(12), 931–936.
Inoue, D., Wada, K., Sei, K., Ike, M., & Fujita, M. (2005). Comparative evaluation of quantitative polymerase chain reaction methods for routine enumeration of specific bacterial DNA in aquatic samples. World Journal of Microbiology and Biotechnology, 21(6), 1029–1035.
Koh, E. G., Huyn, J. H., & Larock, P. A. (1994). Pertinence of indicator organisms and sampling variables to Vibrio concentrations. Applied and Environmental Microbiology, 60(10), 3897–3900.
Lizárraga-Partida M. L., Gómez-Gil, B., Méndez-Gómez, E., Wong-Chang, I., Pardío-Sedas, V., & Cabanillas-Beltrán, H. (2010). Molecular detection of V. cholerae, V. parahaemolyticus and V. vulnificus in oyster from Mexico by the vibriomex group. Vibrios in the Environment 2010. Biloxi, Mississippi, USA.
Luan, X., Chen, J., Liu, Y., Li, Y., Jia, J., Liu, R., & Zhang, X. H. (2008). Rapid quantitative detection of Vibrio parahaemolyticus in seafood by MPN-PCR. Current Microbiology, 57(3), 218–221.
Miwa, N., Nishio, T., Arita, Y., Kawamori, F., Masuda, T., & Akiyama, M. (2003). Evaluation of MPN method combined with PCR procedure for detection and enumeration of Vibrio parahaemolyticus in seafood. Food Hygiene and Safety Science (Shokuhin Eiseigaku Zasshi), 44(6), 289–293.
Rodríguez-Salazar, M. E., Álvarez-Hernández, S., & Bravo-Nuñez, E. (2001). Coeficientes de asociación. México: UAM-I, Plaza y Valdes.
Tantillo, G. M., Fontanarosa, M., Di Pinto, A., & Musti, M. (2004). Updated perspectives on emerging vibrios associated with human infections. Letters in Applied Microbiology, 39(2), 117–126.
Vengadesh, L., Son, R., & Yoke-Kqueen, C. (2012). Molecular quantitation and characterization of Vibrio cholerae from different seafood obtained from wetmarket and supermarket. International Food Research Journal, 19(1), 45–50.
Zar, J. H. (2010). Biostatistical analysis. New Jersey: Pearson Prentice Hall.
Acknowledgments
This research was supported by the following projects: “Molecular detection of pathogenic Vibrio sp. in marine mollusks intended for human consumption, CONACYT-SSA, code number 114024” and “Ecological Integrity Indicators and Environmental Health” (Universidad Autónoma Metropolitana). The experiments were carried out at Universidad Nacional Autónoma de México (UNAM). The authors thank Dr. Hector Barrera Villa Zevallos for the translation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barrera-Escorcia, G., Wong-Chang, I., Fernández-Rendón, C.L. et al. Quantification of Vibrio species in oysters from the Gulf of Mexico with two procedures based on MPN and PCR. Environ Monit Assess 188, 602 (2016). https://doi.org/10.1007/s10661-016-5620-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5620-9