Abstract
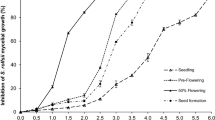
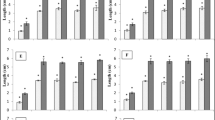
Elicitors are stressors of biotic nature responsible for the adaptation of plants to cope with environmental stresses. Brassicae species (as white mustard) produce glucosinolates (GSs) that modify soil microbial communities and suppress soil-borne plant diseases. This research aimed to evaluate the glucosinolates levels in white mustard after elicitation with salicylic acid (SA), hydrogen peroxide (H2O2), chitosan (QN), and a commercial oligosaccharide (Xh), as well as the impact on the biofumigant activity of elicited plant residues mixed with fresh manure against soil-borne populations of Fusarium oxysporum. White mustard plants were treated with SA, H2O2, QN, and Xh. White mustard residues of the QN treatment (100 mg/mL) were further evaluated either in combination or not with fresh cow manure for biofumigant activity against F. oxysporum. Spectrophotometric and GC–MS techniques, respectively, determined GSs in white mustard plant organs. Significant increases in GSs in all plant organs except roots were found. The best treatment was chitosan 100 mg/mL, showing increases in leaves, flowers, and seeds. These plants treated with chitosan and mixed with fresh cow manure used in the biofumigation of soils displayed a reduction from 1600 CFU/g of soil to 50 CFU/g of soil F. oxysporum native population. Results suggested that controlled elicitation might increase the amount of GSs on white mustard, especially in the aerial parts; thus, elicited white mustard can be valorized as a potent biofumigant when mixed with fresh cow manure; thus, these green amendments might be a sustainable alternative in agriculture to help in crop management.

Similar content being viewed by others
Data Availability
The data that support the findings of this study are not openly available, however they are available from the corresponding author upon reasonable request.
References
Angelino, D., Dosz, E. B., Sun, J., Hoeflinger, J. L., Van Tassell, M. L., Chen, P., Harnly, J. M., Miller, M. J., & Jeffery, E. H. (2015). Myrosinase-dependent and –independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Front in Plant Sci, 6, 831. https://doi.org/10.3389/fpls.2015.00831
Ascencion, L., Wen-Jinn, L., & Tsair-Bor, Y. (2015). Control of Rhizoctonia solani damping-off disease after soil amendment with dry tissues of Brassica results from increase in Actinomycetes population. Biological Control, 82, 21–30. https://doi.org/10.1016/j.biocontrol.2014.11.010
Ayilara, M. S., Olanrewaju, O. S., Babalola, O. O., & Odeyemi, O. (2020). Waste management through composting: Challenges and potentials. Sustainability, 12, 4456. https://doi.org/10.3390/su12114456
Bangarwa, S. K., & Norsworthy, J. K. (2016). Glucosinolate and isothiocyanate production for weed control in plasticulture production system. In J. M. Mérillon, K. Ramawat (Eds.) Glucosinolates. Reference Series in Phytochemistry. (pp. 1–35) Springer, Cham https://doi.org/10.1007/978-3-319-26479-0_9-1
Bennett, R. S. (2012). Survival of Fusarium oxysporum f. sp. vasinfectum chlamydospores under solarization temperatures. Plant Disease, 96(10), 1564-1568.https://doi.org/10.1094/PDIS-09-11-0812-RE
Bohinc, T., Kosir, I., & Trdan, S. (2013). Glucosinolates as arsenal for defending Brassicas against cabbage flea beetle (Phyllotreta spp) attack Zemdirbyste-Agriculture, 100(2), 199–204. https://doi.org/10.13080/z-a.2013.100.026
Bonanomi, G., Alioto, D., Minutolo, M., Marra, R., Cesarano, G., & Vinale, F. (2020). Organic amendments modulate soil microbiota and reduce virus disease incidence in the TSWV-tomato pathosystem. Pathogens, 9(5), 379. https://doi.org/10.3390/pathogens9050379
Bougnom, B. P., & Insam, H. (2009). Ash additives to compost affect soil microbial communities and apple seedling growth. Die Bodenkultur, 60, 5–15.
Cardenas-Manríquez, G., Vega-Muñoz, I., Villagómez-Aranda, A. L., León-Galvan, M. F., Cruz-Hernandez, A., Torres-Pacheco, I., Rangel-Cano, R. M., Rivera-Bustamante, R. F., & Guevara-Gonzalez, R. G. (2016). Proteomic and metabolomic profiles in transgenic tobacco (N. tabacum xanthi nc) to CchGLP from Capsicum chinense BG-3821 resistant to biotic and abiotic stresses. Environmental and Experimental Botany, 130, 33–41. https://doi.org/10.1016/j.envexpbot.2016.05.005
Delgado-Baquerizo, M., Guerra, C. A., Cano-Díaz, C., Egidi, E., Wang, J.-T., Eisenhauer, N., Singh, B. K., & Maestre, F. T. (2020). The proportion of soil-borne pathogens increases with warming at the global scale. Nature Climate Change, 10, 550–554. https://doi.org/10.1038/s41558-020-0759-3
Dudareva, N., & Negre, F. (2005). Practical applications of research into the regulation of plant volatile emission. Current Opinion in Plant Biology, 8(1), 113–118. https://doi.org/10.1016/j.pbi.2004.11.007
Fernández-Bayo, J. D., Randall, T. E., Harrold, D. R., Achmon, Y., Hestmark, K. V., Su, J., Dahlquist-Willard, R. M., Gordon, T. R., Stapleton, J. J., VanderGheynst, J. S., & Simmons, C. W. (2018). Effect of management of organic wastes on inactivation of Brassica nigra and Fusarium oxysporum f sp lactucae using soil biosolarization. Pest Manag Sci, 74(8), 1892–1902.
Gandariasbeitia, M., López-Pérez, J. A., Juaristi, B., & Larregla, S. (2022). Sunflower seed husk as promising by-product for soil biodisinfestation treatments and fertility improvement in protected lettuce crop. Front in Sustain Food Syst, 6, 901654. https://doi.org/10.3389/fsufs.2022.901654
Guo, R., Huang, Z., Deng, Y., Chen, X., XuHan, X., & Lai, Z. (2016). Comparative transcriptome analyses reveal a special glucosinolate metabolism mechanism in Brassica alboglabra Sprouts. Frontiers in Plant Science, 7, 1497. https://doi.org/10.3389/fpls.2016.01497
Hanschen, F. S., & Winkelmann, T. (2020). Biofumigation for fighting replant risease- A Review. Agronomy, 10, 425. https://doi.org/10.3390/agronomy10030425
Hanschen, F. S., Yim, B., Winkelmann, T., Smalla, K., & Schreiner, M. (2015). Degradation of biofumigant isothiocyanates and allyl glucosinolate in soil and their effects on the microbial community composition. PLoS One, 10(7), e0132931. https://doi.org/10.1371/journal.pone.0132931
Hansen, J. C., Schillinger, W. F., Sullivan, T. S., & Paulitz, T. C. (2019). Soil microbial biomass and fungi reduced with canola introduced into long-term monoculture wheat rotations. Front in Microbiol, 10, 1488. https://doi.org/10.3389/fmicb.2019.01488
Haro-Bailón, A., Jurado, A., Pérez-Melgares, J. D., Saavedra, M., Bejarano, J., & Obregón, S. (2013). Variabilidad cualitativa y cuantitativa del contenido en glucosinolatos en especies de crucíferas de interés para la biofumigación del olivar. El Aceite de Oliva. Actas Simposio Expoliva. Jaén (España) 8–11 mayo I.S.B.N. 978–84–938900–1–8
Hartz, T. K., Johnstone, P. R., Miyao, E. M., & Davis, R. M. (2005). Mustard cover crops are ineffective in suppressing soilborne disease or improving processing tomato yield. HortScience, 40(7), 2016–2019. https://doi.org/10.21273/HORTSCI.40.7.2016
Harvey, S., Hannahan, H., & Sams, C. (2002). Indian mustard and allyl isothiocyanate inhibit Sclerotium rolfsii. Journal of the American Society for Horticultural Science., 127(1), 27–31. https://doi.org/10.21273/JASHS.127.1.27
Husna, A. (2020). Fusarium commune associated with wilt and root rot disease in rice. Plant Pathology, 70, 123–132. https://doi.org/10.1111/ppa.13270
Jaime, R., Alcántara, J. M., Manzaneda, A. J., & Rey, P. J. (2018). Climate change decreases suitable areas for rapeseed cultivation in Europe but provides new opportunities for white mustard as an alternative oilseed for biofuel production. PLoS One., 13(11), e0207124. https://doi.org/10.1371/journal.pone.0207124
Jeschke, V., Zalucki, J. M., Raguschke, B., Gershenzon, J., Heckel, D. G., Zalucki, M. P., & Vassão, D. G. (2021). So much for glucosinolates: A generalist does survive and develop on Brassicas, but at what cost? Plants, 10(5), 962. https://doi.org/10.3390/plants10050962
Jezek, J., Haggett, B. G. D., Atkinson, A., & Rawson, D. M. (1999). Determination of glucosinolates using their alkaline degradation and reaction with ferricyanide. Journal of Agricultural and Food Chemistry., 47(11), 4669–4674. https://doi.org/10.1021/jf9906026
Kowalska, J., Tyburski, J., Krzymińska, J., & Jakubowska, M. (2021). Effects of seed treatment with mustard meal in control of Fusarium culmorum Sacc. and the growth of common wheat (Triticum aestivum ssp. vulgare). European Journal of Plant Pathology, 159(3), 327–338. https://doi.org/10.1007/s10658-020-02165-9
Lamondia, J. (1995). Influence of resistant tobacco and tobacco cyst nematodes on root infection and secondary inoculum of Fusarium oxysporum f sp nicotianae. Plant Disease, 79(4), 337–340. https://doi.org/10.1094/PD-79-0337
Lietzow, J. (2021). Biologically active compounds in mustard seeds: A toxicological perspective. Foods, 10, 2089. https://doi.org/10.3390/foods10092089
Martínez-Ballesta, M. D. C., Moreno, D. A., & Carvajal, M. (2013). The physiological importance ofglucosinolates on plant response to abiotic stress in Brassica. International Journal of Molecular Sciences, 14(6), 11607–11625. https://doi.org/10.3390/ijms140611607
Mejía-Teniente, L., Duran-Flores, F. d. D., Chapa-Oliver, A. M., Torres-Pacheco, I., Cruz-Hernández, A., González-Chavira, M. M., Ocampo-Velázquez, R. V., & Guevara-González, R. G. (2013) Oxidative and molecular responses in Capsicum annuum L. after hydrogen peroxide, salicylic acid and chitosan foliar applications. International Journal of Molecular Sciences, 14 5 10178–10196. https://doi.org/10.3390/ijms140510178
Mitrović, P. M., Stamenković, O. S., Banković-Ilić, I., Djalović, I. G., Nježić, Z. B., Farooq, M., Siddique, K. H. M., & Veljković, V. B. (2020). White mustard (Sinapis alba L.) oil in biodiesel production: A Review. Frontiers in Plant Science, 11, 299. https://doi.org/10.3389/fpls.2020.00299
Monaci, E., Casucci, C., De Bernardi, A., Marini, E., Landi, L., Toscano, G., Romanazzi, G., & Vischetti, C. (2022). Brassica carinata seed meal as soil amendment and potential biofumigant. Crops, 2, 233–246. https://doi.org/10.3390/crops2030017
Nguyen, C. H. (2003). Rhizodeposition of organic C by plants: Mechanisms and controls. Agronomie, 23, 375–396. https://doi.org/10.1051/agro:2003011
Njoroge, S. M. C., Riley, M. B., & Keinath, A. P. (2008). Effect of incorporation of Brassica spp. residues on population densities of soilborne microorganisms and on damping-off and Fusarium wilt of watermelon. Plant Diease, 92, 287–294. https://doi.org/10.1094/PDIS-92-2-0287
Oka, Y. (2010). Mechanisms of nematode suppression by organic soil amendments. A Review. Applied Soil Ecology, 44(2), 101–115. https://doi.org/10.1016/j.apsoil.2009.11.003
Okunade, O. A., Ghawi, S. K., Methven, L., & Niranjan, K. (2015). Thermal and pressure stability of myrosinase enzymes from black mustard (Brassica nigra L. W.D.J. Koch. var. nigra), brown mustard (Brassica juncea L. Czern. var. juncea) and yellow mustard (Sinapsis alba L. subsp. maire) seeds. Food Chemistry, 187, 485–490. https://doi.org/10.1016/j.foodchem.2015.04.054
Popova, I. E., & Morra, M. J. (2014). Simultaneous quantification of sinigrin, sinalbin, and anionic glucosinolate hydrolysis products in Brassica juncea and Sinapis alba seed extracts using ion chromatography. Journal of Agricultural and Food Chemistry, 62(44), 10687–10693. https://doi.org/10.1021/jf503755m
Rubayet, M. T., Bhuiyan, K., Jannat, R., Masum, M. M. I., & Hossain, M. (2018). Effect of biofumigation and soil solarization on stem canker and black scurf diseases of potato (Solanum tuberosum L.) caused by Rhizoctonia solani isolate PR2. Advances in Agriculture Sciences, 6(3), 33–48
dos Santos, C. A., de Souza Abboud, A. C., & Goréte Ferreira do Carmo, M. (2021). Biofumigation with species of the Brassicaceae family: A review. Ciência Rural, Santa Maria, v.51:1, e20200440.https://doi.org/10.1590/0103-8478cr2020040
Scheuerell, S., Sullivan, D., & Mahaffee, W. (2005). Suppression of seedling damping-off caused by Pythium ultimum, P irregulare and Rhizoctonia solani in container media amended with a diverse range of Pacific Northwest compost sources. Phytopathology, 95(3), 306–15. https://doi.org/10.1094/PHYTO-95-0306
Suarez-Fernandez, M., Marhuenda-Egea, F. C., Lopez-Moya, F., Arnao, M. B., Cabrera-Escribano, F., Nueda, M. J., Gunsé, B., & Lopez-Llorca, L. V. (2020). Chitosan induces plant hormones and defenses in tomato root exudates. Frontiers in Plant Science, 11, 572087. https://doi.org/10.3389/fpls.2020.572087
Weerakoon, D. M. N., Reardon, C. L., Paulitz, T. C., Izzo, A. D., & Mazzola, M. (2012). Long-term suppression of Pythium abappressorium induced by Brassica juncea seed meal amendment is biologically mediated. Soil Biology & Biochemistry, 51, 44–52. https://doi.org/10.1016/j.soilbio.2012.03.027
Wermter, N. S., Rohn, S., & Hanschen, F. S. (2020). Seasonal variation of glucosinolate hproducts in commercial white and red cabbages (Brassica oleracea var capitate). Foods, 9, 1682. https://doi.org/10.3390/foods9111682
Xie, H., Yan, D., Mao, L., Wang, Q., Li, Y., Ouyang, C., Guo, M., & Cao, A. (2015). Evaluation of methyl bromide alternatives efficacy against soil-borne pathogens nematodes and soil microbial community. PLoS ONE, 10(2), e0117980. https://doi.org/10.1371/journal.pone.0117980
Zhang, D., Yan, D., Fang, W., Huang, B., Wang, X., Wang, X., Zhu, J., Liu, J., Ouyang, C., Li, Y., Wang, Q., & Cao, A. (2019). Chloropicrin alternated with biofumigation increases crop yield and modifies soil bacterial and fungal communities in strawberry production. Science of the Total Environment., 675, 615–622. https://doi.org/10.1016/j.scitotenv.2019.04.222
Zunun-Perez, A. Y., Guevara-Figueroa, T., Jimenez-Garcia, S. N., Feregrino-Perez, A. A., Gautier, F., & Guevara-Gonzalez, R. G. (2017). Effect of foliar application of salicylic acid, hydrogen peroxide and a xyloglucan oligosaccharide on capsiate content and gene expression associated with capsinoids synthesis in Capsicum annuum L. Journal of Biosciences, 42(2), 245–250. https://doi.org/10.1007/s12038-017-9682-9
Acknowledgements
Authors, thanks to Ciencia Basica (CONACyT) and ID4FEED (France) for partial support for this research, D.A.A-M, and A.C-P also thank CONACyT for grant support. Moreover authors thanks to Dr. Gobinath Chandrakasan for checking English style in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vargas-Hernandez, M., Arriaga-Madrid, D.A., Cortez-Perez, A. et al. White mustard (Sinapis alba L.) residues with enhanced potential as soil biofumigant by using controlled elicitation during cultivation. Eur J Plant Pathol 166, 209–218 (2023). https://doi.org/10.1007/s10658-023-02655-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02655-6