Abstract
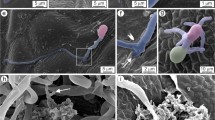
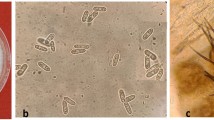
Grape anthracnose, caused by Elsinoë ampelina, is an important fungal disease during grape production. Little is known about the interactions of grape-E.ampelina compared with other grapevine fungal diseases. This study used potted seedlings of Vitis vinifera ‘Red Globe’ and E. ampelina to investigate their interactions under greenhouse conditions. The infection process of E. ampelina in the young leaves of grape was observed from 0 to 6 days post-inoculation (dpi) under microscope. Results showed that the first symptom on grape leaves were observed at 3 dpi. The rates of conidial germination on grape leaves were 23.4, 73.2, and 85.6% at 1, 2, and 3 dpi, respectively. Early penetration occurred in the epidermal cells with or without forming appressoria at 2 dpi. E. ampelina enlarged in intercellular spaces at 2–3 dpi and began to enter intracellular spaces from 3 dpi. New conidia were produced in acervuli on the leaf surface from 6 dpi. The ultrastructural observation revealed defense responses in infected host cells, including collapse of cell walls and chloroplasts, changes in the number of mitochondria, plasmolysis, and cell death. Overall, these results provide a detailed understanding of the grape-E.ampelina interaction and theoretical bases for infection pathway of grape anthracnose.









Similar content being viewed by others
References
Bagsic, I., Linde, M., & Debener, T. (2016). Genetic diversity and pathogenicity of Sphaceloma rosarum (teleomorph Elsinoë rosarum) causing spot anthracnose on roses. Plant Pathology, 65, 978–986.
Becker, M., Becker, Y., Green, K., & Scott, B. (2016). The endophytic symbiont Epichloë festucae establishes an epiphyllous net on the surface of Lolium perenne leaves by development of an expressorium, an appressorium-like leaf exit structure. New Phytologist, 211, 240–254.
Braga, Z. V., Santos, R. F., Amorim, L., & Appezzato-da-Glória, B. (2019). Histopathology of infection and colonisation of Elsinoë ampelina on grapevine leaves. European Journal of Plant Pathology. https://doi.org/10.1007/s10658-019-01721-2.
Brook, P. J. (1973). Epidemiology of grapevine anthracnose, caused by Elsinoë ampelina. New Zealand Journal of Agricultural Research, 16, 333–342.
Coertze, S., Holz, G., & Sadie, A. (2001). Germination and establishment of infection on grape berries by single airborne conidia of Botrytis cinerea. Plant Disease, 85, 668–677.
Cohen, Y., Vaknin, M., Ben-Naim, Y., & Rubin, A. E. (2013). Light suppresses sporulation and epidemics of Peronospora belbahrii. PLoS One, 8, e81282.
Everett, K. R., Rees-George, J., Pushparajah, I. P., Manning, M. A., & Fullerton, R. A. (2011). Molecular identification of Sphaceloma perseae (avocado scab) and its absence in New Zealand. Journal of Phytopathology, 159, 106–113.
Fan, X. L., Barreto, R. W., Groenewald, J. Z., Bezerra, J. D. P., Pereira, O. L., Cheewangkoon, R., Mostert, L., Tian, C. M., & Crous, P. W. (2017). Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Studies in Mycology, 87, 1–41.
Gao, M., Wang, Q., Wan, R., Fei, Z., & Wang, X. (2012). Identification of genes differentially expressed in grapevine associated with resistance to Elsinoë ampelina through suppressive subtraction hybridization. Plant Physiology and Biochemistry, 58, 253–268.
Gao, Y. R., Han, Y. T., Zhao, F. L., Li, Y. J., Cheng, Y., Ding, Q., Wang, Y. J., & Wen, Y. Q. (2016). Identification and utilization of a new Erysiphe necator isolate NAFU1 to quickly evaluate powdery mildew resistance in wild Chinese grapevine species using detached leaves. Plant Physiology and Biochemistry, 98, 12–24.
Gilbert, R. D., Johnson, A. M., & Dean, R. A. (1996). Chemical signals responsible for appressorium formation in the rice blast fungus Magnaporthe grisea. Physiologyical and Molecular Plant Pathology, 48, 335–346.
Guest, D., Brown, J. (1997). Infection processes. In: Brown, J. F., ogle, H. J., eds. Plant pathogens and plant diseases. pp 245–262.
Hyun, J. W., Yi, S. H., MacKenzie, S. J., Timmer, L. W., Kim, K. S., Kang, S. K., Kwon, H. M., & Lim, H. C. (2009). Pathotypes and genetic relationship of worldwide collections of Elsinoë spp. causing scab disease of citrus. Phytopathology, 99, 721–728.
Jang, M. H., Ahn, S. Y., Kim, S. H., Noh, J. H., & Yun, H. K. (2011). Evaluation of grapevine varietal resistance to anthracnose through treating culture filtrates from Elsinoe ampelina. Horticulture, Environment, and Biotechnology, 52, 152–157.
Jayasankar, S., Li, Z., & Gray, D. J. (2000). In-vitro selection of Vitis vinifera 'Chardonnay' with Elsinoë ampelina culture filtrate is accompanied by fungal resistance and enhanced secretion of chitinase. Planta, 211, 200–208.
Kim, K. W., Hyun, J. W., & Park, E. W. (2004). Cytology of cork layer formation of citrus and limited growth of Elsinoe fawcettii in scab lesions. European Journal of Plant Pathology, 110, 129–138.
Kobae, Y., Ohmori, Y., Saito, C., Yano, K., Ohtomo, R., & Fujiwara, T. (2016). Phosphate treatment strongly inhibits new arbuscule development but not the maintenance of arbuscule in mycorrhizal rice roots. Plant Physiology, 171, 566–579.
Kono, A., Sato, A., Ban, Y., & Mitani, N. (2013). Resistance of Vitis germplasm to Elsinoë ampelina (de Bary) shear evaluated by lesion number and diameter. HortScience, 48, 1433–1439.
Lam, E., Kato, N., & Lawton, M. (2001). Programmed cell death, mitochondria and the plant hypersensitive response. Nature, 411, 848–853.
Li, Z., Dang, H., Yuan, X., He, J., Hu, Z., & Wang, X. (2018). Morphological characterization and optimization of conditions for conidial production of Elsinoë ampelina, the causal organism of grapevine anthracnose. Journal of Phytopathology, 166, 420–428.
Liao, H. L., & Chung, K. R. (2008). Cellular toxicity of elsinochrome phytotoxins produced by the pathogenic fungus, Elsinoë fawcettii causing citrus scab. New Phytologist, 177, 239–250.
Magarey, R. D., Emmett, R. W., Magarey, P. A., & Franz, P. R. (1993). Evaluation of control of grapevine anthracnose caused by Elsinoë ampelina by pre-infection fungicides. Australasian Plant Pathology, 22, 48–52.
Paudyal, D. P., & Hyun, J. W. (2015). Physical changes in Satsuma mandarin leaf after infection of Elsinoë fawcettii causing citrus scab disease. Plant Pathology Journal, 31, 421–427.
Paudyal, D. P., Hyun, J. W., & Hwang, R. Y. (2017). Infection and symptom development by citrus scab pathogen Elsinoë fawcettii on leaves of satsuma mandarin. European Journal of Plant Pathology, 148, 807–816.
Podila, G. K., Rogers, L. M., & Kolattukudy, P. E. (1993). Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiology, 103, 267–272.
Poolsawat, O., Tharapreuksapong, A., Wongkaew, S., Chaowiset, W., & Tantasawat, P. (2012). Laboratory and field evaluations of resistance to Sphaceloma ampelinum causing anthracnose in grapevine. Australasian Plant Pathology, 41, 263–269.
Rumbolz, J., & Gubler, W. D. (2005). Susceptibility of grapevine buds to infection by powdery mildew Erysiphe necator. Plant Pathology, 54, 535–548.
Ryder, L. S., & Talbot, N. J. (2015). Regulation of appressorium development in pathogenic fungi. Current Opinion in Plant Biology, 26, 8–13.
Santos, R. F., & Spósito, M. B. (2018). Improving assessments of anthracnose severity on grapevine leaves through the development of a standard area diagram set. Australasian Plant Pathology, 47, 357–364.
Santos, R. F., Ciampi-Guillardi, M., Amorim, L., Massola Júnior, N. S., & Spósito, M. B. (2018a). Aetiology of anthracnose on grapevine shoots in Brazil. Plant Pathology, 67, 692–706.
Santos, R. F., Spósito, M. B., Ayres, M. R., & Sosnowski, M. R. (2018b). Phylogeny, morphology and pathogenicity of Elsinoë ampelina, the causal agent of grapevine anthracnose in Brazil and Australia. Journal of Phytopathology, 166, 187–198.
Santos, R. F., Spósito, M. B., Ayres, M., & Sosnowski, M. (2018c). In vitro production of conidia of Elsinoë ampelina, the causal fungus of grapevine anthracnose. European Journal of Plant Pathology, 152, 815–821.
Scheper, R. W., Wood, P. N., & Fisher, B. M. (2013). Isolation, spore production and Koch’s postulates of Elsinoe pyri. New Zealand Plant Protection, 66, 308–316.
Stickens, D., & Verbelen, J. P. (1996). Spatial structure of mitochondria and ER denotes changes in cell physiology of cultured tobacco protoplasts. Plant Journal, 9, 85–92.
Su, Y. Y., Qi, Y. L., & Cai, L. (2012). Induction of sporulation in plant pathogenic fungi. Mycology, 3, 195–200.
Tanaka, E., Kumagawa, T., Ito, N., Nakanishi, A., Ohta, Y., Suzuki, E., Adachi, N., Hamada, A., Ashizawa, T., Ohara, T., & Tsuda, M. (2017). Colonization of the vegetative stage of rice plants by the false smut fungus Villosiclava virens, as revealed by a combination of species-specific detection methods. Plant Pathology, 66, 56–66.
Thind, T. (2015). Anthracnose. In: Wilcox W, Gubler W, Uyemoto J. (eds) Compendium of grape diseases, disorders, and pests, 2nd edn. St Paul, MN, USA, APS Press, pp 17–19.
Vesty, E. F., Saidi, Y., Moody, L. A., Holloway, D., Whitbread, A., Needs, S., Choudhary, A., Burns, B., McLeod, D., Bradshaw, S. J., Bae, H., Chrisopher, K., Bassel, G. W., Simonsen, H. T., & Coates, J. C. (2016). The decision to germinate is regulated by divergent molecular networks in spores and seeds. New Phytologist, 211, 952–966.
Wan, R., Hou, X., Wang, X., Qu, J., Singer, S. D., Wang, Y., & Wang, X. (2015). Resistance evaluation of Chinese wild Vitis genotypes against Botrytis cinerea and different responses of resistant and susceptible hosts to the infection. Frontiers in Plant Science, 6, 854.
Wang, Y., Liu, Y., He, P., Lamikanra, O., & Lu, J. (1998). Resistance of Chinese Vitis species to Elsinoë ampelina (de Bary) shear. HortScience, 33, 123–126.
Wang, X., McCalluma, B. D., Fetcha, T., Bakkerenb, G., Maraisa, G. F., & Saville, B. J. (2013). Comparative microscopic and molecular analysis of Thatcher near-isogenic lines with wheat leaf rust resistance genes Lr2a, Lr3, LrB or Lr9 upon challenge with different Puccinia triticina races. Plant Pathology, 62, 698–707.
Williamson, B., & McNicol, R. J. (1989). The histology of lesion development in raspberry canes infected by Elsinoe veneta. Annals of Applied Biology, 114, 35–44.
Yan, H. Q., Zhang, T. T., Lan, S. C., & Jiang, S. (2018). Ultrastructural study on the interaction between Physcomitrella patens and Botrytis cinerea. Plant Pathology, 67, 42–50.
Yin, X., Liu, R. Q., Su, H., Su, L., Guo, Y. R., Wang, Z. J., Du, W., Li, M. J., Zhang, X., Wang, Y. J., Liu, G. T., & Xu, Y. (2017). Pathogen development and host responses to Plasmopara viticola in resistant and susceptible grapevines: An ultrastructural study. Horticulture Research, 4, 17033.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31501740 and 31572110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest. All authors fully agree for submission of the manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Fig. S1
Appressorium of E. ampelina on the leaves of V. vinifera ‘Red Globe’ at 48 hpi. C, conidia; Is, infection site; App, appressorium. Scale bar = 10 μm. (JPG 70 kb)
Fig. S2
Mitochondrial morphology in uninoculated leaves of V. vinifera ‘Red Globe’. Ch, chloroplast; Cw, cell wall; M, mitochondria. Scale bar = 2 μm. (JPG 116 kb)
Rights and permissions
About this article
Cite this article
Li, Z., Zhang, S., Han, R. et al. Infection process and host responses to Elsinoë ampelina, the causal organism of grapevine anthracnose. Eur J Plant Pathol 155, 571–582 (2019). https://doi.org/10.1007/s10658-019-01793-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01793-0