Abstract
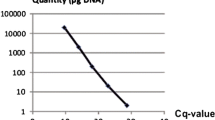
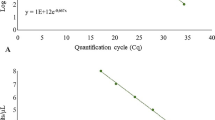
The high-temperature-tolerant Pythium species P. aphanidermatum, P. helicoides, and P. myriotylum cause serious diseases in many crops under hydroponic culture systems in Japan. Control of the diseases is difficult because these zoosporic pathogens spread quickly. In this study, a real-time PCR method was developed for monitoring the spread of zoospores of the three pathogens. Specific primers and TaqMan probes were established using the internal transcribed spacer regions of the rDNA. Specificity was confirmed using known isolates of each species and closely related non-target species. The sensitivity of DNA detection was 10 f. for each pathogen. 10 f. DNA corresponded to 4 P. aphanidermatum, 3 P. myriotylum, and 4 P. helicoides zoospores, respectively. Therefore, this real-time PCR method was used to evaluate and monitor zoospores in the nutrient solutions of ebb-and-flow irrigation systems for potted flower production and closed hydroponic culture systems for tomato production. The results indicated that the pathogens were present in the hydroponic culture systems throughout the year, and spread before disease occurrence.



Similar content being viewed by others
References
Asano, T., Kageyama, K., & Hyakumachi, M. (1999). Surface disinfestation of resting spores of Plasmodiophora brassicae used to infect hairy roots of Brassica spp. Phytopathology, 89, 314–319.
Asano, T., Senda, M., Suga, H., & Kageyama, K. (2010). Development of multiplex PCR to detect five Pythium species related to turf-grass diseases. Journal of Phytopathology, 158, 609–615.
Bridge, P. D., Arora, D. K., Reddy, D. K., Elander, R. P., (Ed.), CAB International, New York. (1998). Interpretation of PCR methods for species definition. Applications of PCR in Mycology, 63–84
Chen, W. (1992). Restriction fragment length polymorphisms in enzymatically amplified ribosomal DNAs of three heterothallic Pythium species. Phytopathology, 82, 1467–1472.
Chilvers, M. I., du Toit, L. J., Akamatsu, H., & Peever, T. L. (2007). A real-time, quantitative PCR seed assay for Botrytis spp. that cause neck rot of onion. Plant Disease, 91, 599–608.
Edel, V., Bridge, P. D., Arora, D. K., Reddy, C. A., Elander, R. P., (Ed.), CAB international, New York. (1998). Polymerase chain reaction in mycology. Applications of PCR in Mycology, 1–20
Hong, C. X., & Moorman, G. W. (2005). Plant pathogens in irrigation water: challenges and opportunities. Critical Reviews in Plant Sciences, 24, 189–208.
Hong, C., Richardson, P. A., & Kong, P. (2002). Comparison of membrane filters as a tool for isolating Pithiaceous species from irrigation water. Phytopathology, 92, 610–616.
Ishiguro, Y., Asano, T., Otsubo, K., Suga, H., & Kageyama, K. (2013). Simultaneous detection by multiplex PCR of the high-temperature-growing Pythium species: P. aphanidermatum, P. helicoides and P. myriotylum. Journal of General Plant Pathology, 79, 350–358.
Kageyama, K. (2011). Characteristics of high-temperature-growing Pythium species now-a-days frequently occurring disease. Plant Protect, 65, 32–26.
Kageyama, K., Aoyagi, T., Sunouchi, R., & Fukui, H. (2002). Root rot of miniature roses caused by Pythium helicoides. Journal of General Plant Pathology, 68, 15–20.
Kageyama, K., Komatsu, T., & Suga, H. (2003a). Refined PCR protocol for detection of plant pathogens in soil. Journal of General Plant Pathology, 69, 153–160.
Kageyama, K., Suzuki, M., Priyatmojo, A., Oto, Y., Ishiguro, K., Suga, H., Aoyagi, T., & Fukui, H. (2003b). Characterization and identification of asexual strains of Pythium associated with root rot of rose in Japan. Journal of Phytopathology, 151, 485–491.
Kageyama, K., Ishiguro, Y., Otsubo, K., Suzuki, H., Tsuji, T., Hashizume, F., Fujita, A., Suga, H. (2013). Monitoring of Pythium aphanidermatum and P. myriotylum in Nutrient Solution of Hydroponic Culture of Tomato Using Real Time PCR. Japanese Journal of Phytopathology (in press).
Klassen, G. R., Balcerzak, M., & de Cock, W. A. M. (1996). 5S ribosomal RNA gene spacers as species-specific probes for eight species of Pythium. Phytopathology, 86, 581–587.
Kontanis, E. J., & Reed, F. A. (2006). Evaluation of real-time PCR amplification efficiencies to detect PCR inhibitors. Journal of Forensic Sciences, 51, 795–804.
Lévesque, C. A., & de Cock, W. A. M. (2004). Molecular phylogeny and taxonomy of the genus Pythium. Mycological Research, 108, 1363–1383.
Li, M., Asano, T., Suga, H., & Kageyama, K. (2011). A multiplex PCR for the detection of Phytophthora nicotianae and P. cactorum and a survey of their occurrence in strawberry production areas of Japan. Plant Disease, 95, 1270–1278.
Li, M., Inada, M., Watanabe, H., Suga, H., & Kageyama, K. (2013). Simultaneous detection and quantification of Phytophthora nicotianae and P. cactorum, and distribution analyses in strawberry greenhouses by duplex real-time PCR. Microbes and Environments, 28(2), 195–203.
Mackay, I. M., Arden, K. E., & Nitsche, A. (2002). Real-time PCR in virology. Nucleic Acids Research, 30, 1292–1305.
McCartney, H. A., Foster, S. J., Fraaije, B. A., & Ward, E. (2003). Molecular diagnostics for fungal plant pathogens. Pest Management Science, 59, 129–142.
Meherun, N., Motohash, K., Watanabe, H., Chikuo, Y., Senda, M., Suga, H., Brasier, C., & Kageyama, K. (2011). Phytophthora chrysanthemi sp. nov., a new species causing root rot of chrysanthemum in Japan. Mycological Progress, 10, 21–31.
Miyake, N., Nagai, H., Kageyama, K. (2014) Wilting and root rot of poinsettia caused by three high-temperature-tolerant Pythium species in ebb-and-flow irrigation systems. Journal of General Plant Pathology , In press
Nielsen, C. J., Ferrin, D. M., & Stanghellini, M. E. (2006). Efficacy of biosurfactants in the management of Phytophthora capsici on pepper in recirculating hydroponic systems. Can J Plant Pathol Rev Can Phytopathologie, 28, 450–460.
Pettitt, T. R., Wakeham, A. J., Wainwright, M. F., & White, J. G. (2002). Comparison of serological, culture and bait methods for detection of Pythium and Phytophthora zoospores in water. Plant Pathology, 51, 720–727.
Postma, J., Geraats, B. P. J., Pastoor, R., & van Elsas, J. D. (2005). Characterization of the microbial community involved in the suppression of Pythium aphanidermatum in cucumber grown on rockwool. Phytopathology, 95, 808–818.
Schroeder, K. L., Martin, F. N., de Cock, A. W. A. M., Lévesque, C. A., Spies, C. F. J., Okubara, P. A., & Paulitz, T. C. (2013). Molecular detection and quantification of Pythium species: evolving taxonomy, new tools, and challenges. Plant Disease, 97, 4–20.
Suzuki, M., Togawa, M., & Yoneyama, C. (2005). Occurrence of Pythium root rot of strawberry caused by Pythium helicoides. Jpn J Phytopathology, 71, 209.
Suzuki, H., Tsuji, T., Hashizume, H., Fujita, T., & Kuroda, K. (2013). Population dynamics of high-temperature-growing Pythium species on tomato in hydroponic and effects of water tempareture on incidence. Jpn J Phytopathology, 79, 58.
Szalanski, A. L., Sui, D. D., Harris, T. S., & Powers, T. O. (1997). Identification of cyst nematodes of agronomic and regulatory concern with PCR-RFLP of ITS 1. Journal of Nematology, 29, 255–267.
Thelwell, N., Millington, S., Solinas, A., Booth, J., & Brown, T. (2000). Mode of action and application of Scorpion primers to mutation detection. Nucleic Acids Research, 28, 3752–3761.
van der Plaats-Niterink, A. J. (1981). Monograph of the genus Pythium. Centraalbureau voor Schimmelcultures. Stud Mycol, 21, 1–242.
Wang, P. H., & Chang, C. W. (2003). Detection of the low-germinationrate resting oospores of Pythium myriotylum from soil by PCR. Letters in Applied Microbiology, 36, 157–161.
Watanabe, T. (1977). Pathogenicity of Pythium myriotylum isolated from strawberry roots in Japan. Ann Phytopathol Soc Jpn, 43, 306–309.
Watanabe, H., Horinouchi, H., Tanahashi, I., & Kageyama, K. (2005). Occurrence of root rot of strawberry caused by Pythium helicoides, and pathogenicity to several crops. Jpn J Phytopathology, 71, 209–210.
Watanabe, H., Taguchi, Y., Horinouchi, H., Hyakumachi, M., & Kageyama, K. (2007). Pythium and Phytophthora species associated with root and stem rots of KALANCHOE. Journal of General Plant Pathology, 73, 81–88.
Waterhouse, G. M. (1967). Key to Pythium Pringsheim. Commonw Mycol Inst Mycol Pap, 109, 1–15.
White, T. J., Bruns, T., Lee, S., Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols, 315–322. A Guide to Methods and Applications. Innis, M. A., Gelgard, D. H., Sninsky, J. J., White, T. J., (Ed.), Academic Press, New York.
Acknowledgment
This research was funded by the development of diagnostic manual of high-temperature tolerant Pythium species in hydroponic culture system project for application in promoting new policy of Japanese Ministry of Agriculture Forestry and Fisheries. We thank Dr. M. D. Coffey, and Dr. S. Uematsu for providing isolates of Phytophthora species.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Ishiguro, Y., Otsubo, K. et al. Monitoring by real-time PCR of three water-borne zoosporic Pythium species in potted flower and tomato greenhouses under hydroponic culture systems. Eur J Plant Pathol 140, 229–242 (2014). https://doi.org/10.1007/s10658-014-0456-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-014-0456-z