Abstract
Musculoskeletal conditions, including fractures, can have severe and long-lasting consequences. Higher body mass index in adulthood is widely acknowledged to be protective for most fracture sites. However, sources of bias induced by confounding factors may have distorted previous findings. Employing a lifecourse Mendelian randomisation (MR) approach by using genetic instruments to separate effects at different life stages, this investigation aims to explore how prepubertal and adult body size independently influence fracture risk in later life.
Using data from a large prospective cohort, univariable and multivariable MR were conducted to simultaneously estimate the effects of age-specific genetic proxies for body size (n = 453,169) on fracture risk (n = 416,795). A two-step MR framework was additionally applied to elucidate potential mediators. Univariable and multivariable MR indicated strong evidence that higher body size in childhood reduced fracture risk (OR, 95% CI: 0.89, 0.82 to 0.96, P = 0.005 and 0.76, 0.69 to 0.85, P = 1 × 10− 6, respectively). Conversely, higher body size in adulthood increased fracture risk (OR, 95% CI: 1.08, 1.01 to 1.16, P = 0.023 and 1.26, 1.14 to 1.38, P = 2 × 10− 6, respectively). Two-step MR analyses suggested that the effect of higher body size in childhood on reduced fracture risk was mediated by its influence on higher estimated bone mineral density (eBMD) in adulthood.
This investigation provides novel evidence that higher body size in childhood reduces fracture risk in later life through its influence on increased eBMD. From a public health perspective, this relationship is complex since obesity in adulthood remains a major risk factor for co-morbidities. Results additionally indicate that higher body size in adulthood is a risk factor for fractures. Protective effect estimates previously observed are likely attributed to childhood effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Musculoskeletal conditions are a leading cause of disability worldwide, affecting nearly 2 billion people [1]. Associated injuries, including fractures and falls, can lead to serious and long-lasting effects, particularly in later life [2]. With hip fracture predicted to incur an annual worldwide cost of US$132 billion by 2050 [3], not only the social, but economic burden of these health states make prevention of such conditions an important public health goal [4].
Considerable changes occur in body composition over the lifecourse [5]. Bone mass is accrued until peak bone mass is reached in the third decade of life [6]. It then remains stable until menopause in women, and later life in men, where sex steroid deficiency begins to drive cortical bone loss [7, 8]. Lean mass additionally increases during growth in childhood, remaining largely stable following puberty until falling later in life [9]. Conversely, body fat tends to rise in older age groups, with obesity prevalence peaking in individuals aged 60 to 69 years in high-income countries [10, 11]. Several meta-analyses have pointed to a complex relationship between body mass index (BMI) and fracture risk [12,13,14]. Although higher BMI in adulthood is widely acknowledged to be a protective factor for most sites of fragility fracture [12, 13], studies have shown conflicting results with some evidence suggesting that obesity may be related to an increased risk of fracture [14, 15]. Mendelian randomisation (MR) exploits the quasi-random assortment of genetic variants independent of other traits to mitigate against false inferences resulting from confounding and reverse causality [16,17,18]. Using the principles of MR, previous investigations have indicated that higher adiposity increases bone mineral density (BMD) in childhood [16] and that low BMD increases the risk of fracture [19]. Moreover, a population-based birth cohort study supported by a subsequent MR investigation identified fat mass as a positive determinant of bone mass and size in prepubertal children [20, 21], pointing to the positive effects of loading on bone formation at a young age [16]. Fat mass may stimulate bone growth in childhood through a direct mechanical action of increased load [22], or indirectly by association with increased lean mass, since both are strongly correlated across the whole range of body mass [23]. It is therefore plausible that the protective effect estimates observed between higher BMI in adulthood and fracture risk in later life, could be attributed to the effects of higher body size that may have exerted an influence on the skeleton in childhood.
Separating the effects of body size at different stages of the lifecourse is challenging, particularly due to the influence of confounding factors, often afflicting conventional epidemiological studies. This is a key motivation behind using a lifecourse MR approach, which intends to estimate the causal effect of time-varying modifiable risk factors under specific assumptions; the instrumental variables used must (i) associate with the exposure of interest conditional on the other exposures (the ‘relevance’ assumption), (ii) not affect the outcome except through the exposures (the ‘exclusion restriction’ assumption) and (iii) be independent of all confounders, both observed and unobserved, of the instrumental variable and the outcome (the ‘exchangeability’ assumption) [24, 25]. The core aim of this investigation was to apply this approach to explore how weight-based body size (henceforth body size) at two different stages in the lifecourse modifies the risk of fractures in later life.
Materials and methods
Data resources
Genetic variants strongly associated with childhood and adult body size (using P < 5 × 10− 8 and r2 < 0.001) were identified in a large-scale genome-wide association study (GWAS), previously undertaken in the UK Biobank study on 453,169 individuals, adjusting for age, sex, and genotyping chip [26, 27]. UK Biobank data were collected between 2006 and 2010 on individuals aged between 40 and 69 years old at baseline, from clinical examinations, assays of biological samples, detailed information on self-reported health characteristics, and genome-wide genotyping, using a prospective cohort study design [27]. The childhood body size measure applied in this study, utilised recall questionnaire data, involving responses from adult participants who were asked whether, compared to the average, they were ‘thinner’, ‘about average’ or ‘plumper’, when they were aged 10 years old. The adult body size variable was derived using clinically measured body mass index (BMI) data (mean age 56.5 years). It was then separated into a 3-tier variable using the same categories as the childhood body size measure; “thinner” (21.1 kg/m2-25 kg/m2), “about average” (25 kg/m2-31.7 kg/m2) and “plumper” (31.7 kg/m2-59.9 kg/m2). Individuals that did not have data for both childhood and adult body size were excluded from analyses and a genetic correlation coefficient of rG = 0.61 was previously calculated between these two measures [30]. In addition, these scores have been independently validated in two distinct cohorts, providing verification that these genetic instruments can reliably separate childhood and adult body size [28, 29]. Furthermore, comparing the genetic correlation between the childhood body size GWAS with a recent GWAS of measured childhood BMI, provided strong evidence of validation using LD score regression (rg = 0.96) [31].
For the main outcome, fractures in later life, a previously published GWAS on individuals in the UK Biobank was used (n = 416,795) [32]. This excluded fractures of the skull, face, hands and feet, pathological fractures due to malignancy, atypical femoral fractures, periprosthetic, and healed fracture codes and a full list of ICD10 codes used have been reported previously [32]. Effect estimates derived from results indicate an additive change in the odds of each change in weight category in childhood and adult body size [30]. To generate genetic instruments for birthweight (n = 261,932), GWAS were undertaken on UK Biobank individuals with adjustment for gestational age, sex and genotyping chip. We used a linear mixed model to account for genetic relatedness and geographical structure in UK Biobank as undertaken with the BOLT-LMM software. Birthweight was kept as a continuous trait given that it was not available in the full sample and rank-based inverse normal transformed, to ensure values lay within accepted limits assuming a normal distribution. We additionally ran GWAS for several potential mediators using UK Biobank data with the application of the same analysis pipeline stipulated above. Mediators included genetic predisposition to increased serum calcium in nmol/L (n = 432,151), vitamin D level in nmol/L (n = 449,913) and BMD estimated by quantitative ultrasound of the heel calcaneus (hereafter, “eBMD”) (n = 278,932), in the total population as well as bioavailable testosterone (female n = 206,604, male n = 207,470), total testosterone (female n = 180,386, male n = 184,025), and SHBG (female n = 222,491, male n = 205,646), in the sex-stratified population. We standardised the distribution of these variables to have a mean of 0 and standard deviation of 1. Results are quantified as standard deviation change. In addition, in sensitivity analyses this study estimated effects on sex (n = 361,194) to assess participation bias, as well as whole body fat mass (n = 453,957) and whole-body fat-free mass (n = 454,669) which were measured in kilograms and made into indices (kg/m2) by dividing by height (m2) using UK Biobank data (S1 Table).
Conducting MR using overlapping sets of participant samples has been shown to bias in the direction of results generated from conventional epidemiological analyses between the risk factor and outcome [33]. We therefore used previously developed formulae implemented in a web application (https://sb452.shinyapps.io/overlap/) to calculate expected bias and Type 1 error rate under the null for genetically proxied childhood body size and odds of fracture in later life [34]. Data used to generate output is in S2 Table. Estimated bias due to sample overlap is presented in S3 Table.
The UK Biobank study have obtained ethics approval from the Research Ethics Committee (REC; approval number: 11/NW/0382) and informed consent from all participants enrolled in UK Biobank. Estimates were derived using data from the UK Biobank (app #76538).
Statistical analysis
Univariable MR was initially conducted to estimate the ‘total’ effects of genetically predicted childhood body size and adult body size on fractures in later life. Firstly, the inverse variance weighted method (IVW) was employed, which takes SNP-outcome estimates and regresses them on the SNP-exposure associations (Fig. 1A) [35]. A test for heterogeneity was subsequently conducted for this analysis. Complementary methods, namely weighted median and MR-Egger were used to assess the robustness of the univariable results to horizontal pleiotropy, whereby genetic variants influence multiple traits or disease outcomes via independent biological pathways [36]. Multivariable IVW MR, an extension of MR that employs multiple genetic variants associated with multiple measured risk factors, was used to calculate the direct and indirect effects of childhood body size and adult body size, simultaneously, on fractures in later life, accounting for either adult body size or childhood body size, respectively i.e., the exposure variables that were not considered the main exposure of interest in each model (Fig. 1B) [24, 25]. Genetic estimates for our exposures were harmonized with mediators and the disease outcome using the ‘TwoSampleMR’ R package. Forest plots in this paper were generated using the R package ‘ggplot2’ [37]. These analyses were undertaken using R (version 3.5.1).
Two-step mendelian randomisation
To investigate the possible mechanisms by which body size affects fractures and determine potential intermediate traits, we applied the principles of MR in a two-step framework [38]. This was achieved by, (i) assessing the separate effects of genetically predicted childhood body size and adult body size on each of the potential non-sex-specific mediators; serum calcium and vitamin D levels, as well as eBMD in adulthood, as outcomes, and then, (ii) assessing each of these mediators as exposures on the outcome, fractures in later life (Fig. 1C). We then ran multivariable IVW MR to estimate the indirect effect of childhood body size on fracture risk accounting for eBMD to ascertain whether this effect is mediated by eBMD [39]. Multivariable IVW MR analyses were additionally computed to calculate the direct and indirect effects of childhood body size and adult body size, simultaneously, on mediators of interest.
Sex-stratification
Given the literature, suggesting that effects of weight are often sex-specific [40], we explored these questions separately by sex. We examined three hormones (bioavailable testosterone, total testosterone, and sex hormone binding globulin (SHBG)) shown to be related to BMI and bone health, as potential mediators between childhood body size and both fracture risk in later life and eBMD, in the sex-stratified population [41,42,43,44]. Univariable and multivariable MR analyses were conducted in sex-stratified groups; female or male, assigned at birth. Two-sample MR was subsequently computed to determine any potential hormonal sex-specific mechanisms of action between body size and eBMD as well as fractures in later life.
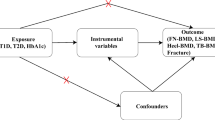
Directed acyclic graphs indicating three scenarios to explain the causal effect between childhood body size and fracture risk in later life. (A) Univariable Mendelian randomization measuring the total effect of body size in childhood on the odds of fracture in later life. (B) Multivariable Mendelian randomization measuring the direct and indirect effect of body size in childhood and adulthood on the odds of fracture in later life. (C) Two-step Mendelian randomization measuring the total effect of body size in childhood on the mediator (Step 1) and the total effect of the mediator on the odds of fracture in later life (Step 2), allowing the measurement of the indirect effect of body size in childhood on the odds of fracture in later life via the mediator, e.g., estimated bone mineral density
Sensitivity analyses
Investigating birthweight as a third exposure
Birthweight was investigated as a third exposure related to body size in (i) univariable MR, assessing the ‘total’ effect of birthweight on the outcome, fractures in later life, (ii) multivariable MR assessing the ‘direct’ and ‘indirect’ effect of birthweight on the outcome, by taking childhood and adult body size into account, and (iii) in both childhood and adult body size multivariable MR models, accounting for birthweight, to determine whether any effects of childhood and adult body size observed are a result of weight in very early life [45]. A combination of foetal- and maternal-specific mechanisms and tissues have been identified in the regulation of birthweight, with some mechanisms involving directionally opposing effects in the foetus and mother [46]. Therefore, this investigation of birthweight was not to determine the effects of parental factors on fracture risk in later life, but to exclude the possibility that very early life body size is an explanation of the childhood body size effect.
Body composition measures
To investigate whether results indicate a true causal effect of childhood body size, that does not discriminate between adiposity and lean mass, on fracture risk in later life, we explored the effects of childhood and adult body size on fat mass index and fat-free mass index in a univariable and multivariable MR setting. In addition, we conducted multivariable MR analyses to assess the relationship between childhood body size and fracture risk in later life, accounting for fat mass index and fat-free mass index to estimate potential mediation. This was also to maintain an estimate with suitable comparability to the adult measure used (a genetic proxy for BMI).
Sex-differential participation bias
We investigate the potential for artefactual associations as a result of sex-differential participation, whereby childhood or adult body size may have led males and females to differentially participate in the UK Biobank study. This was achieved through estimating variants associated with the traits, childhood and adult body size, and sex [47].
Family-based analyses
Findings from MR analyses of unrelated individuals may be biased as a result of uncontrolled confounding from familial effects: dynastic effects, assortative mating, or population stratification [48]. It has been argued that within-family genetic association estimates, for example, those acquired from samples of siblings, may allow more accurate estimates of direct genetic effects since these are unaffected by demography and indirect genetic effects of parents [49]. Population (between-family) and within-sibship (within-family) estimates (n = 39,507) were therefore generated to examine the extent to which the direction of our estimates could be influenced by dynastic effects, assortative mating, or population stratification.
Results
Univariable analyses indicated evidence that higher genetically predicted childhood body size reduced the odds of fractures in later life (IVW OR, 95% CI: 0.89, 0.82 to 0.96, P = 0.005). Multivariable MR analyses showed strong evidence that higher genetically predicted childhood body size reduced the odds of fractures in later life, after accounting for adult body size (OR, 95% CI: 0.76, 0.69 to 0.85, P = 1 × 10− 6). There was some evidence that higher genetically predicted adult body size increased the odds of fractures in later life (OR, 95% CI: 1.08, 1.01 to 1.16, P = 0.023). There was additionally strong evidence that higher genetically predicted adult body size increased the odds of fractures in later life, after accounting for childhood body size (OR, 95% CI: 1.26, 1.14 to 1.38, P = 2 × 10− 6) (Table 1; Fig. 2). Multivariable MR analyses additionally showed little evidence that higher genetically predicted childhood body size reduced the odds of fractures, accounting for eBMD (OR, 95% CI: 0.99, 0.90 to 1.08, P = 0.762). Findings from the two-sample MR weighted median and MR-Egger methods revealed similar trends to IVW estimates. This suggests little evidence that horizontal pleiotropy is driving our results, with the exception of adult body size on the odds of fractures using MR-Egger (Table 1).
Two-step mendelian randomisation
Among the mediators assessed in the total population, strong evidence of an effect was observed in both MR steps for childhood and adult body size and eBMD. An increase per one standard deviation in genetically predicted childhood and adult body size indicated an increase in eBMD (beta, 95% CI: 0.26, 0.20 to 0.33, P = 3 × 10− 16 and 0.20, 0.16 to 0.25, P = 1 × 10− 17, respectively). In multivariable MR, the magnitude of the effect estimate slightly weakened when estimating childhood body size on eBMD (beta, 95% CI: 0.20, 0.12 to 0.27, P = 3 × 10− 7) and substantially when estimating adult body size on eBMD (beta, 95% CI: 0.09, 0.02 to 0.15, P = 0.008) (Fig. 3). In addition, an increase in eBMD was predicted to decrease fracture risk in later life (OR, 95% CI: 0.62, 0.60 to 0.64, P = 5 × 10− 150) (S4 Table).
There was strong evidence that higher genetically predicted childhood body size decreased serum calcium (beta, 95% CI: -0.11, -0.15 to -0.06, P = 6 × 10− 7), and evidence that higher genetically predicted adult body size decreased serum calcium (beta, 95% CI: -0.05, -0.09 to -0.01, P = 0.006), however, little evidence that an increase in serum calcium decreased the odds of fractures in later life (OR, 95% CI: 0.99, 0.95 to 1.04, P = 0.813). Furthermore, there was strong evidence that higher genetically predicted childhood and adult body size decreased vitamin D (beta, 95% CI: -0.07, -0.10 to -0.04, P = 5 × 10− 5 and − 0.18, -0.21 to -0.16, P = 1 × 10− 37) and little evidence that an increase in vitamin D increased the odds of fractures in later life (OR, 95% CI: 1.02, 0.96 to 1.09, P = 0.541) (S4 Table; Fig. 4).
Sex-stratification
Upon stratification, there was strong evidence that higher childhood body size increased eBMD in females (beta, 95% CI: 0.18, 0.10 to 0.26, P = 7 × 10− 6) and in males (beta, 95% CI: 0.36, 0.23 to 0.49, P = 3 × 10− 8). After accounting for adult body size, there remained evidence of an increase in eBMD with central effect estimates more comparable between females and males (beta, 95% CI: 0.21, 0.13 to 0.29, P = 5 × 10− 7 and beta, 95% CI: 0.20, 0.05 to 0.346, P = 0.008, respectively). There was additionally strong evidence of an increase in eBMD and decrease in fractures in later life in females (OR, 95% CI: 0.60, 0.57 to 0.63, P = 2 × 10− 112) and males (OR, 95% CI: 0.69, 0.65 to 0.73, P = 5 × 10− 40) (S4 Table). Consistent patterns of associations were observed using the weighted median method employed for robustness. In addition, results using the MR-Egger method did not provide evidence that horizontal pleiotropy was responsible for the estimates derived. Two-step MR estimates computed to investigate bioavailable testosterone, total testosterone, and SHBG, as potential mechanisms of action between body size and fractures in later life are presented in Fig. 5. Further estimates from two-sample analyses, are presented in S5 Table.
Sensitivity analyses
Investigating birthweight as a third exposure
In univariable analyses, there was some evidence that higher genetically predicted birthweight increased the odds of fractures in later life (OR, 95% CI: 1.08, 1.01 to 1.16, P = 0.036). In multivariable analyses, there was evidence that higher genetically predicted birthweight increased the odds of fractures in later life (OR, 95% CI: 1.03, 1.01 to 1.17, P = 0.003), accounting for childhood and adult body size. There was strong evidence that higher childhood body size increased the odds of fractures in later life, after accounting for adult body size and birthweight (OR, 95% CI: 0.78, 0.69 to 0.88, P = 7 × 10− 5). There was additionally strong evidence that higher genetically precited adult body size increased the odds of fractures in later life (OR, 95% CI: 1.21, 1.09 to 1.35, P = 3 × 10− 4), after accounting for childhood body size and birthweight (S6 Table).
Body composition measures
There was strong evidence that higher genetically predicted childhood and adult body size increased fat-free mass index (kg/m2), measured in adulthood (beta, 95% CI: 0.74, 0.69 to 0.79, P = 3 × 10− 195 and 1.00, 0.98 to 1.02, P < 1 × 10− 300, respectively). Consistent patterns of associations were observed using the weighted median method employed for robustness and results using the MR-Egger method did not provide evidence that horizontal pleiotropy was responsible for the derived estimates (S7 Table). In multivariable MR analyses, whilst the beta reduced, there remained strong evidence that genetically predicted childhood body size increased fat-free mass index, after accounting for adult body size (beta, 95% CI: 0.18, 0.14 to 0.21, P = 8 × 10− 23). There was additionally strong evidence that higher genetically predicted adult body size increased fat-free mass index, after accounting for childhood body size (beta, 95% CI: 0.91, 0.88 to 0.94, P < 1 × 10− 300). Furthermore, in univariable analyses, there was strong evidence of an effect between higher childhood and adult body size and increased fat mass index (kg/m2), measured in adulthood (beta, 95% CI: 0.78, 0.72 to 0.84, P = 7 × 10− 154 and 1.30, 1.29 to 1.32, P < 1 × 10− 300, respectively). In multivariable MR after accounting for adult body size, there was strong evidence that higher genetically predicted childhood body size reduced fat mass index (beta, 95% CI: -0.05, -0.08 to -0.02, P = 5 × 10− 4). There remained strong evidence of an effect between higher genetically predicted adult body size and increase in fat mass index, after accounting for childhood body size (beta, 95% CI: 1.33, 1.30 to 1.35, P = 1 × 10− 300). Further estimates, including those stratified by sex, are in S7 Table.
The relationship between childhood body size and the odds of fracture in later life increased marginally, after accounting for fat-free mass index (OR, 95% CI: 0.82, 0.73 to 0.92, P = 0.001) and reduced after accounting for fat mass index (OR, 95% CI: 0.76, 0.68 to 0.84, P = 0.008) (S8 Table). This suggests that the childhood body size measure used in this analysis does not discriminate between adiposity and lean mass and may therefore provide a more accurate depiction of what the measure of BMI attempts to capture.
Sex-differential participation bias
There was evidence of an association between childhood and adult body size on sex. For example, results indicate that childhood and adult body size reduced the odds of being female (OR, 95% CI: 0.94, 0.89 to 0.99, P = 0.029 and OR, 95% CI: 0.96, 0.91 to 1.00, P = 0.049, respectively) (S9 Table). These artifactual relationships observed suggest some sex-differential participation bias is apparent.
Family-based analyses
Between-family univariable and multivariable estimates reveal consistent directions of effect with estimates from the whole population. Results are underpowered, however. This is due to (i) the large reduction in the sample size, from the whole population (n = 501,550) to a subset of siblings in the UK Biobank (n = 39,507) and (ii) there being difference in genetics in between-sibling analyses (S10 Table).
Discussion
In this MR study, we employed a lifecourse framework to evaluate the effects of genetically proxied childhood and adult body size on fractures in later life. We observed strong evidence that higher childhood body size reduced fracture risk in later life. This effect became stronger after accounting for adult body size. Conversely, we identified strong evidence that higher adult body size increased the odds of fracture in univariable as well as multivariable analyses, after accounting for childhood body size. Findings from this study suggest that higher body size in childhood may have a lasting influence on fracture risk in later life and therefore, the protective effect estimates observed in previous clinical and conventional epidemiological research between BMI in adults and fracture risk [12, 13], are likely attributed to childhood effects. Where greater body size leads to an adaptive change in bone size and strength during growth, our results suggest that this does not occur in later life once growth has ceased. Importantly, the childhood body size measure used in this investigation does not discriminate between adipose and lean mass, or between fat stored in different compartments of the body. Furthermore, investigating birthweight as a third exposure showed that the effects of childhood and adult body size observed were not a result of body size at birth.
We additionally investigated whether plausible risk factors for fractures served as intermediate variables (mediators) on the causal pathway between childhood body size and fracture risk in later life using two-step MR. An increase in genetically predicted childhood body size was strongly associated with a decrease in serum calcium and vitamin D levels and an increase in eBMD in adulthood. There was, however, very little evidence of an effect between vitamin D and serum calcium on the odds of fracture in later life. On the other hand, we observed a strong causal association between an increase in eBMD and a decrease in the odds of fracture risk. Indeed, adiposity in childhood has been shown previously to be causally related to BMD in childhood, specifically of the limbs, pelvis, and spine, and not the skull [16]. One hypothesis is that this reflects the positive effects of loading on bone formation at weighted sites. Since eBMD is derived from ultrasound of the calcaneum, it primarily represents a measure of trabecular bone. As such, the effect we show between childhood body size and eBMD suggests that body size may affect the amount of trabecular bone. As well as there being a scaling relationship between body size and overall bone size during growth, the internal bone structure is positively influenced by body size. This appears to persist throughout life, protecting against fracture risk regardless of BMI reduction in adulthood. In addition, whilst calcium and vitamin D supplementation is recommended for fracture prevention [50,51,52], findings from randomised clinical trials yield conflicting conclusions regarding their efficacy [53,54,55]. Common variants in PTHR1, a gene that regulates calcium ion homeostasis, are also shown to influence BMD and height variation in populations through effects on bone mass acquisition [56], However, our results are supported by recent findings from MR studies suggesting that genetically predicted lower levels of vitamin D do not appear to be associated with fracture risk and genetically predicted higher levels of serum calcium levels do not improve eBMD [19, 57, 58].
Since effects of body weight are often sex-specific [40], we investigated the relationship between childhood body size and fracture risk as well as childhood body size and eBMD, separately by sex. The strength of the genetically predicted effect of childhood body size in males compared to females was more than 2-fold in magnitude on both outcome measures. Upon accounting for adult body size, these differences diminished, suggesting that our adult body size measure was, to a large degree, responsible for the sex differences observed in the outcomes of interest. These results are in line with previous research and highlight a complex relationship between sex hormone profiles, body size and bone health [59, 60]. Furthermore, higher childhood body size was strongly associated with a decrease of bioavailable testosterone in males and increase in females. This finding is supported by the literature, whereby obese males have been characterised by a decrease in testosterone levels with increasing body weight [61]. Their female counterparts, conversely, have been shown to develop a condition of functional hyperandrogenism [62], which is, in most cases, detectable by testosterone elevation [61]. Reduced SHBG synthesis and circulating blood levels have also been shown to represent the sole common mechanism response for this in both males and females [61], with the former observed in our findings as additionally occurring in response to higher body size in childhood. There was also strong evidence that bioavailable testosterone reduced the risk of fractures in both males and females. These associations, again, were stronger in males than they were in females. Furthermore, since the sex hormone measures used in this study were quantified in an adult population (mean age: 56.5 years), it is likely their effect is more strongly related to adult body size. This is in line with the literature, which has shown age-related testosterone deficiency to be the most important factor of bone loss in elderly men [63] and that SHBG in midlife is linked with injury risk in both sexes [41]. It is additionally plausible, that the sex-differential associations observed in this study are, in part, a result of sex-differential participation bias, where the determinants of study participation affect females and males to differing extents [47]. Evidence of this has been shown where artifactual associations between variants associated with childhood and adult body size and sex were observed.
From a public health perspective, the relationship between BMI and fracture risk is complex, as obesity in adulthood remains a major risk factor for co-morbidities, including diabetes, cardiovascular diseases, cancer, and other health problems that may lead to further morbidity and mortality [64]. On the other hand, childhood BMI does not have independent risk increasing effects on coronary heart disease [65] or type 2 diabetes [30], for example, and is additionally protective against breast cancer [66]. It is, however, of importance to quantify the effect of BMI at different stages in the lifecourse on the risk of fracture in later life, to help (i) identify modifiable pathways to fracture risk to decipher potential intervention targets, and (ii) enhance the predictive value of BMI at different time points in fracture risk case finding [13, 64]. In addition, this investigation highlights the importance of processes operating across the lifecourse that influence the development of risk in later life [67].
Strengths and limitations
Investigations have previously assessed the relationship between BMI and fracture risk in conventional epidemiological analyses, as well as cross-sectionally, using MR methods. This is a unique study in that it estimates the effects of body size on fracture risk at separate timepoints in the lifecourse. At the same time, it infers causality by utilising the relationship between genetic variants robustly associated with a modifiable exposure or biological intermediate of interest and a disease outcome. An important and perhaps underreported methodological limitation in much of the obesity literature is through the use of BMI as an imperfect measure of adiposity [23]. Whilst BMI indicates overweight relative to height, it does not discriminate between adiposity and lean mass. Our study explores this measure to conclude that it is both indicative of adiposity and lean mass. Our research additionally revealed potential mediators on the causal pathway between childhood body size and fracture risk, to aid in the identification of prospective intervention targets that may help to reduce fracture risk in later life. Furthermore, this investigation was able to leverage large sample sizes available through the UK Biobank study (n = 453,169) for all measures used, by calculating expected bias and Type 1 error rate under the null that could result from using overlapping samples. In addition, weighted median and MR-Egger methods were used to assess the robustness of univariable results to horizontal pleiotropy.
This study, however, also has important limitations. First, self-reporting of body size in childhood by participants may have led to differential social desirability bias, in relation to retrospective weight recall at age 10. Moreover, the age of participants in adulthood when reporting this information could have influenced this measurement. To account for this, GWAS were computed on individuals who had both measures available adjusting for age, as well as sex and the genotyping chip. Second, our measure of childhood body size did not discriminate between adipose and lean mass, nor did it between fat stored in different compartments of the body. Third, using sex hormones quantified in adulthood, as opposed to childhood where sex hormone levels are substantially different, limited our ability to decipher potentially important mechanisms between childhood body size and sex hormone regulation at the same timepoint. We additionally used fat and lean mass measures form adulthood, which likely associated more strongly with adult BMI than childhood as a result. Fourth, another important sex-hormone that requires further investigation within this research area is oestradiol, however, sample sizes are currently much lower than those for other sex-hormones in the UK Biobank (female n = 53,391, male n = 17,134). Fifth, we were only able to examine the role of eBMD in terms of how bone parameters mediate effects of body size on fracture risk due to the availability of measures on bone architecture. Including aspects related to bone size in future research would be helpful in separating the mechanisms by which body size affects fracture risk in children and adults. Sixth, in using the UK Biobank, selection bias is a central limitation. Participation in the UK Biobank has been shown to be associated with being older, female and living in areas that are less likely to be socioeconomically deprived than individuals in nationally representative data sources [68]. Therefore, this analysis is under-representative of younger, male, non-binary or any other gender identity individuals as well as those from the lowest socioeconomic groups. This has the potential to result in problems for instrumental variable analyses [69]. In addition, we have shown that our sex-stratified results may, in part, exhibit artefactual autosomal heritability in the presence of sex-differential participation bias, which has been shown to lead to incorrect inferences in downstream analyses [47]. Moreover, since allele frequencies as well as risk factors and diseases vary between subgroups in the population, confounding is plausible. This study thus performs analyses in homogeneous populations of European ancestry [70], therefore only depicting effects within this single ancestry group that may not be generalisable to other ancestry populations. Future research would benefit from replicating this across a broader range of different ancestries.
Conclusion
This investigation provides novel evidence that higher genetically proxied childhood body size has a direct effect on reduced fractures in later life, via increased eBMD. From a public health perspective, this relationship is complicated since adulthood obesity remains a major risk factor for co-morbidities including diabetes, cardiovascular diseases, cancer, and other health problems. Conversely, higher childhood BMI does not have independent risk increasing effects on coronary heart disease or type 2 diabetes and is protective against breast cancer. Findings may additionally help to identify modifiable pathways to fracture risk to decipher potential intervention targets as well as enhance the predictive value of BMI at different time points in fracture risk case finding. Further work is required to investigate this in more detail. Importantly, results additionally indicate that higher BMI in adulthood is indeed a risk factor for fractures, opposing earlier clinical and conventional epidemiological research findings which denote it as protective. The protective effect estimates previously observed between higher BMI in adults and fracture risk are therefore likely attributed to childhood effects.
References
Csuhai ÉA, Nagy AC, Szőllősi GJ, Veres-Balajti I. Impact Analysis of 20-Week Multimodal Progressive Functional-Proprioceptive Training among Sedentary Workers Affected by Non-Specific Low-Back Pain: An Interventional Cohort Study.Int J Environ Res Public Health. 2021;18(20).
Pineles SL, Repka MX, Yu F, Lum F, Coleman AL. Risk of musculoskeletal injuries, fractures, and falls in medicare beneficiaries with disorders of binocular vision. JAMA Ophthalmol. 2015;133(1):60–5.
Johnell O. The socioeconomic burden of fractures: today and in the 21st century. Am J Med. 1997;103(2a):20S-5S; discussion 5S-6S.
Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in Community-Dwelling older adults: a systematic review and Meta-analysis. JAMA. 2017;318(24):2466–82.
Zheng R, Byberg L, Larsson SC, Höijer J, Baron JA, Michaëlsson K. Prior loss of body mass index, low body mass index, and central obesity independently contribute to higher rates of fractures in elderly women and men. J Bone Miner Res. 2021;36(7):1288–99.
Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729–39.
Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–70.
Farr JN, Khosla S. Skeletal changes through the lifespan–from growth to senescence. Nat Rev Endocrinol. 2015;11(9):513–21.
Siervogel RM, Demerath EW, Schubert C, Remsberg KE, Chumlea WC, Sun S, et al. Puberty and body composition. Horm Res. 2003;60(Suppl 1):36–45.
Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26(7):953–60.
Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32(9):1431–7.
Johansson H, Kanis JA, Odén A, McCloskey E, Chapurlat RD, Christiansen C, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223–33.
De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330–8.
Palermo A, Tuccinardi D, Defeudis G, Watanabe M, D’Onofrio L, Lauria Pantano A, et al. BMI and BMD: the potential interplay between obesity and bone fragility. Int J Environ Res Public Health. 2016;13(6):544.
Gonnelli S, Caffarelli C, Nuti R. Obesity and fracture risk. Clin Cases Miner Bone Metab. 2014;11(1):9–14.
Kemp JP, Sayers A, Davey Smith G, Tobias JH, Evans DM. Using mendelian randomization to investigate a possible causal relationship between adiposity and increased bone mineral density at different skeletal sites in children. Int J Epidemiol. 2016;45(5):1560–72.
Richmond RC, Davey Smith G. Mendelian randomization: concepts and scope. Cold Spring Harbor Perspectives in Medicine; 2021.
Davey Smith G, Ebrahim S. Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22.
Trajanoska K, Morris JA, Oei L, Zheng H-F, Evans DM, Kiel DP, et al. Assessment of the genetic and clinical determinants of fracture risk: genome wide association and mendelian randomisation study. BMJ. 2018;362:k3225.
Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91(7):2534–41.
Timpson NJ, Sayers A, Davey Smith G, Tobias JH. How does body fat influence bone mass in childhood? A mendelian randomization approach. J Bone Miner Res. 2009;24(3):522–33.
Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9(1):87–97.
Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC, et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. 2018;362:k2575.
Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48(3):713–27.
Sanderson E, Glymour MM, Holmes MVK, Hyunseung, Morrison J, Munafò MR, Palmer T, et al. Mendelian randomization. Nat Reviews Methods Primers. 2022;2(1):7.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–9.
Brandkvist M, Bjørngaard JH, Ødegård RA, Åsvold BO, Davey Smith G, Brumpton B, et al. Separating the genetics of childhood and adult obesity: a validation study of genetic scores for body mass index in adolescence and adulthood in the HUNT study. Hum Mol Genet. 2021;29(24):3966–73.
Richardson TG, Mykkänen J, Pahkala K, Ala-Korpela M, Bell JA, Taylor K et al. Evaluating the direct effects of childhood adiposity on adult systemic metabolism: a multivariable Mendelian randomization analysis.Int J Epidemiol. 2021.
Richardson TG, Sanderson E, Elsworth B, Tilling K, Davey Smith G. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: mendelian randomisation study. BMJ. 2020;369:m1203.
Vogelezang S, Bradfield JP, Ahluwalia TS, Curtin JA, Lakka TA, Grarup N, et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 2020;16(10):e1008718.
Morris JA, Kemp JP, Youlten SE, Laurent L, Logan JG, Chai RC, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. 2019;51(2):258–66.
Nelson CR, Startz R. The distribution of the instrumental variables estimator and its t-Ratio when the instrument is a poor one. J Bus. 1990;63(1):125–S40.
Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample mendelian randomization. Genet Epidemiol. 2016;40(7):597–608.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Ginestet C. ggplot2: elegant graphics for data analysis. J R Stat Soc a Stat. 2011;174:245.
Relton CL, Davey Smith G. Two-step epigenetic mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. 2012;41(1):161–76.
Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465–78.
Rask-Andersen M, Karlsson T, Ek WE, Johansson Ã. Genome-wide association study of body fat distribution identifies adiposity loci and sex-specific genetic effects. Nat Commun. 2019;10(1):339.
Leinonen JT, Mars N, Lehtonen LE, Ahola-Olli A, Ruotsalainen S, Lehtimäki T et al. Genetic analyses on the health impacts of testosterone highlight effects on female-specific diseases and sex differences.medRxiv. 2021:2021.04.23.21255981.
Eriksson J, Haring R, Grarup N, Vandenput L, Wallaschofski H, Lorentzen E, et al. Causal relationship between obesity and serum testosterone status in men: a bi-directional mendelian randomization analysis. PLoS ONE. 2017;12(4):e0176277.
Mohammadi-Shemirani P, Chong M, Pigeyre M, Morton RW, Gerstein HC, Paré G. Effects of lifelong testosterone exposure on health and disease using Mendelian randomization.Elife. 2020;9.
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–8.
Yu XH, Wei YY, Zeng P, Lei SF. Birth weight is positively associated with adult osteoporosis risk: observational and mendelian randomization studies. J Bone Miner Res. 2021;36(8):1469–80.
Warrington NM, Beaumont RN, Horikoshi M, Day FR, Helgeland Ø, Laurin C, et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat Genet. 2019;51(5):804–14.
Pirastu N, Cordioli M, Nandakumar P, Mignogna G, Abdellaoui A, Hollis B, et al. Genetic analyses identify widespread sex-differential participation bias. Nat Genet. 2021;53(5):663–71.
Brumpton B, Sanderson E, Heilbron K, Hartwig FP, Harrison S, Vie G, et al. Avoiding dynastic, assortative mating, and population stratification biases in mendelian randomization through within-family analyses. Nat Commun. 2020;11(1):3519.
Howe LJ, Nivard MG, Morris TT, Hansen AF, Rasheed H, Cho Y et al. Within-sibship GWAS improve estimates of direct genetic effects.bioRxiv. 2021:2021.03.05.433935.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8.
Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–66.
Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–9.
Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83.
Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330(7498):1003.
Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257–64.
Vilariño-Güell C, Miles LJ, Duncan EL, Ralston SH, Compston JE, Cooper C, et al. PTHR1 polymorphisms influence BMD variation through Effects on the growing Skeleton. Calcif Tissue Int. 2007;81(4):270–8.
Sun JY, Zhang H, Zhang Y, Wang L, Sun BL, Gao F, et al. Impact of serum calcium levels on total body bone mineral density: a mendelian randomization study in five age strata. Clin Nutr. 2021;40(5):2726–33.
Cerani A, Zhou S, Forgetta V, Morris JA, Trajanoska K, Rivadeneira F, et al. Genetic predisposition to increased serum calcium, bone mineral density, and fracture risk in individuals with normal calcium levels: mendelian randomisation study. BMJ. 2019;366:l4410.
Cooper C, Kuh D, Egger P, Wadsworth M, Barker D. Childhood growth and age at menarche. Br J Obstet Gynaecol. 1996;103(8):814–7.
Ito M, Yamada M, Hayashi K, Ohki M, Uetani M, Nakamura T. Relation of early menarche to high bone mineral density. Calcif Tissue Int. 1995;57(1):11–4.
Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520.
Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319–40.
Mohamad N-V, Soelaiman I-N, Chin K-Y. A concise review of testosterone and bone health. Clin Interv Aging. 2016;11:1317–24.
Chan RSM, Woo J. Prevention of overweight and obesity: how effective is the current public health approach. Int J Environ Res Public Health. 2010;7(3):765–83.
Power GM, Tyrrell J, Frayling TM, Davey Smith G, Richardson TG. Mendelian randomization analyses suggest childhood body size indirectly influences end points from across the Cardiovascular Disease Spectrum through adult body size. J Am Heart Assoc. 2021;10(17):e021503.
Vabistsevits M, Davey Smith G, Sanderson E, Richardson TG, Lloyd-Lewis B, Richmond RC. Deciphering how early life adiposity influences breast cancer risk using mendelian randomization. Commun Biol. 2022;5(1):337.
Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Community Health. 2003;57(10):778–83.
Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of Sociodemographic and Health-Related characteristics of UK Biobank participants with those of the General Population. Am J Epidemiol. 2017;186(9):1026–34.
Hughes RA, Davies NM, Davey Smith G, Tilling K. Selection Bias when estimating average treatment Effects using one-sample Instrumental Variable Analysis. Epidemiology. 2019;30(3):350–7.
Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an Approach to assess causality using Observational Data. J Am Soc Nephrol. 2016;27(11):3253–65.
Acknowledgements
We would like to thank the UK Biobank study and all participants who contributed to it, as well as the authors of all the GWAS who made their summary statistics available for the benefit of this work. This research has been conducted using the UK Biobank Resource under Application Number 76538.
Funding
This work was in part supported by the Integrative Epidemiology Unit which receives funding from the UK Medical Research Council and the University of Bristol (MC_UU_00011/1). GDS conducts research at the NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. GMP is supported by the GW4 Biomed Doctoral Training Programme, awarded to the Universities of Bath, Bristol, Cardiff and Exeter from the Medical Research Council (MRC)/UKRI (MR/N0137941/1). TGR was a UKRI Innovation Research Fellow whilst contributing to this study (MR/S003886/1). TMF has received funding from the Medical Research Council (MR/T002239/1) EU-IMI SOPHIA and GSK. JT is supported by an Academy of Medical Sciences (AMS) Springboard award, which is supported by the AMS, the Wellcome Trust, GCRF, the Government Department of Business, Energy and Industrial strategy, the British Heart Foundation and Diabetes UK (SBF004\1079). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: George Davey Smith, Tom G. Richardson; Methodology: Grace Marion Power; Formal analysis and investigation: Grace Marion Power; Writing - original draft preparation: Grace Marion Power; Writing - review and editing: Jonathan H Tobias, Timothy M. Frayling, Jessica Tyrrell, April E. Hartley, Jon E. Heron, George Davey Smith, Tom G. Richardson; Funding acquisition: George Davey Smith; Supervision: George Davey Smith, Tom G. Richardson.
Corresponding author
Ethics declarations
Competing interests
I have read the journal’s policy and the authors of this manuscript have the following competing interests: TGR is an employee of GlaxoSmithKline outside of the work presented in this manuscript.
Ethics approval
The UK Biobank study have obtained ethics approval from the Research Ethics Committee (REC; approval number: 11/NW/0382).
Consent to participate
The UK Biobank study have obtained informed consent from all participants enrolled in UK Biobank. Estimates were derived using data from the UK Biobank (app #76538).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Power, G.M., Tobias, J.H., Frayling, T.M. et al. Age-specific effects of weight-based body size on fracture risk in later life: a lifecourse Mendelian randomisation study. Eur J Epidemiol 38, 795–807 (2023). https://doi.org/10.1007/s10654-023-00986-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-023-00986-6