Abstract
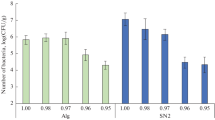
The Salar del Hombre Muerto is a flat salt with great microbial activity despite the existing extreme conditions like high altitude, lack of water, low level of oxygen, high radiation and high concentration of sodium and lithium chloride. Despite these unfavorable conditions, we found microbial diversity with the presence of fungi, algae, and bacteria. From aqueous solutions and soil samples, a total of 238 bacteria were isolated and 186 of them were able to grow in the presence of salt. About 30% of the strains showed the ability to grow in solid medium proximally to a LiCl solution close to saturation (636 g/L). These isolates were characterized taking into account the morphology, Gram stain, ability to form biofilms and to produce pigments, and mainly according to the tolerance against lithium chloride. Bacillus was predominant among the most tolerant 26 microorganisms found, followed by Micrococcus and Brevibacterium. Members of the genera Kocuria, Curtobacterium and Halomonas were also represented among the bacteria with tolerance to 30 and 60 g/L of LiCl in defined liquid medium. All the capacities found in these microorganisms make them extremely interesting for biotechnological applications.



Similar content being viewed by others
References
Abrol, I. P., Yadav, J. S. P. & Massoud, F. I. (1988). Salt-affected soils and their management. Rome: Food and Agriculture Organization of the United Nations (FAO) http://www.fao.org/docrep/x5871e/x5871e00.htm#Contents. Accessed 14 June 2017.
Ali, I., Prasongsuk, S., Akbar, A., Aslam, M., Lotrakul, P., Punnapayak, H., et al. (2016). Hypersaline habitats and halophilic microorganisms. Maejo International Journal of Science and Technology, 10(3), 330–345.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2.
Amjres, H., Béjar, V., Quesada, E., Abrini, J., & Llamas, I. (2011). Halomonas rifensis sp. nov., an exopolysaccharide producing, halophilic bacterium isolated from a solar saltern. International Journal of Systematic and Evolutionary Microbiology, 61(11), 2600–2605. https://doi.org/10.1099/ijs.0.027268-0.
Amoozegar, M. A., Ghazanfari, N., & Didari, M. (2012). Lead and cadmium bioremoval by Halomonas sp., an exopolysaccharide-producing halophilic bacterium. Progress in Biological Sciences, 2(1), 1–11.
Arias, D., Cisternas, L. A., & Rivas, M. (2017). Biomineralization of calcium and magnesium crystals from seawater by halotolerant bacteria isolated from Atacama Salar (Chile). Desalination, 405, 1–9. https://doi.org/10.1016/j.desal.2016.11.027.
Arora, S., Vanza, M. J., Mehta, R., Bhuva, C., & Patel, P. N. (2014). Halophilic microbes for bio-remediation of salt affected soils. African Journal of Microbiology Research, 8(33), 3070–3078. https://doi.org/10.5897/AJMR2014.6960.
Belfiore, C., Curia, M. V., & Farías, M. E. (2017). Characterization of Rhodococcus sp. A5wh isolated from a high altitude Andean lake to unravel the survival strategy under lithium stress. Revista Argentina de Microbiología. https://doi.org/10.1016/j.ram.2017.07.005.
Bui, E. N. (2013). Soil salinity: A neglected factor in plant ecology and biogeography. Journal of Arid Environments, 92, 14–25.
Chen, G., Chen, X., Yang, Y., Hay, A. G., Yu, X., & Chen, Y. (2011). Sorption and distribution of copper in unsaturated Pseudomonas putida CZ1 biofilms as determined by X-ray fluorescence microscopy. Applied and Environmental Microbiology, 77(14), 4719–4727. https://doi.org/10.1128/AEM.00125-11.
Chien, C. C., Lin, B. C., & Wu, C. H. (2013). Biofilm formation and heavy metal resistance by an environmental Pseudomonas sp. Biochemical Engineering Journal, 78, 132–137.
Dalmaso, G. Z. L., Ferreira, D., & Vermelho, A. B. (2015). Marine extremophiles a source of hydrolases for biotechnological applications. Marine Drugs, 13, 1925–1965. https://doi.org/10.3390/md13041925.
DasSarma, S. & DasSarma, P. (2017). Halophiles. In: eLS. Chichester: John Wiley & Sons, Ltd. https://doi.org/10.1002/9780470015902.a0000394.pub4
Dhakar, K., & Pandey, A. (2016). Wide pH range tolerance in extremophiles: Towards understanding an important phenomenon for future biotechnology. Applied Microbiology and Biotechnology, 100(6), 2499–2510. https://doi.org/10.1007/s00253-016-7285-2.
Eaton, A. D., & American Public Health Association; American Water Works Association; Water Environment Federation. (2005). Standard Methods for the examination of water and wastewaters (21st ed.). Washington, DC: APHA-AWWA-WEF.
Edbeib, M. F., Wahab, R. A., & Huyop, F. (2016). Halophiles: Biology, adaptation, and their role in decontamination of hypersaline environments. World Journal of Microbiology & Biotechnology, 32(8), 135. https://doi.org/10.1007/s11274-016-2081-9.
Fasahati, P., Woo, E. C., & Liu, J. J. (2015). Industrial-scale bioethanol production from brown algae: Effects of pretreatment processes on plant economics. Applied Energy, 139, 175–187.
Fukuchi, S., Yoshimune, K., Wakayama, M., Moriguchi, M., & Nishikawa, K. (2003). Unique amino acid composition of proteins in halophilic bacteria. Journal of Molecular Biology, 327(2), 347–357. https://doi.org/10.1016/S0022-2836(03)00150-5.
Gómez, P. I., Barriga, A., Cifuentes, A. S., & González, M. A. (2003). Effect of salinity on the quantity and quality of carotenoids accumulated by Dunaliella salina (strain CONC-007) and Dunaliella bardawil (strain ATCC 30861) chlorophyta. Biological Research, 36(2), 185–192. https://doi.org/10.4067/S0716-97602003000200008.
Goswami, D., Pithwa, S., Dhandhukia, P., & Thakker, J. N. (2014). Delineating Kocuria turfanensis 2M4 as a credible PGPR: A novel IAA-producing bacteria isolated from saline desert. Journal of Plant Interactions, 9(1), 566–576.
Gürtler, V., & Stanisich, V. (1996). New approaches to typing and identification of bacteria using the 16s–23s rDNA spacer region. Microbiology, 142, 3–16.
Irazusta, V., Nieto-Peñalver, C. G., Cabral, M. E., Amoroso, M. J., & Castellanos de Figueroa, L. I. (2013). Relationship among carotenoid production, copper bioremediation and oxidative stress in Rhodotorula mucilaginosa RCL-11. Process Biochemistry, 48(5–6), 803–809.
Isbell, R. (1996). The Australian soil classification. Melbourne: CSIRO Publishing.
Iyer, A., Mody, K., & Jha, B. (2005). Biosorption of heavy metals by a marine bacterium. Marine Pollution Bulletin, 50(3), 340–343. https://doi.org/10.1016/j.marpolbul.2004.11.012.
Jin, Y., Weining, S., & Nevo, E. (2005). A MAPK gene from Dead Sea fungus confers stress tolerance to lithium salt and freezing-thawing: Prospects for saline agriculture. Proceedings of the National Academy of Sciences of the United States of America, 102(52), 18992–18997. https://doi.org/10.1073/pnas.0509653102.
Lima, R. N., & Porto, A. L. (2016). Recent advances in marine enzymes for biotechnological processes. Advances in Food and Nutrition Research, 78, 153–192. https://doi.org/10.1016/bs.afnr.2016.06.005.
Lowe, B. A., Marsh, T. L., Isaacs-Cosgrove, N., Kirkwood, R. N., Kiupel, M., et al. (2011). Microbial communities in the tonsils of healthy pigs. Veterinary Microbiology, 147(3–4), 346–357. https://doi.org/10.1016/j.vetmic.2010.06.025.
Ma, Y., Galinski, E. A., Grant, W. D., Oren, A., & Ventosa, A. (2010). Halophiles 2010: Life in saline environments. Applied and Environmental Microbiology, 76(21), 6971–6981. https://doi.org/10.1128/AEM.01868-10.
Majzlik, P., Strasky, A., Adam, V., Němec, M., Trnkova, L., Zehnalek, J., et al. (2011). Influence of zinc (II) and copper (II) ions on streptomyces bacteria revealed by electrochemistry. International Journal of Electrochemical Science, 6, 2171–2191.
Mandelli, F., Miranda, V. S., Rodrigues, E., & Mercadante, A. Z. (2012). Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World Journal of Microbiology & Biotechnology, 28(4), 1781–1790.
Mata, J. A., Béjar, V., Llamas, I., Arias, S., Bressollier, P., Tallon, R., et al. (2006). Exopolysaccharides produced by the recently described halophilic bacteria Halomonas ventosae and Halomonas anticariensis. Research in Microbiology, 157(9), 827–835. https://doi.org/10.1016/j.resmic.2006.06.004.
Merritt, J. H., Kadouri, D. E., & O’Toole, G. A. (2011). Growing and analyzing static biofilms. Current Protocols in Microbiology. https://doi.org/10.1002/9780471729259.mc01b01s22.
Mishra, D., Kim, D. J., Ralph, D. E., Ahn, J. G., & Rhee, Y. H. (2008). Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Management, 28(2), 333–338. https://doi.org/10.1016/j.wasman.2007.01.010.
Moraga, N. B., Poma, H. R., Amoroso, M. J., & Rajal, V. B. (2014). Isolation and characterization of indigenous Streptomyces and Lentzea strains from soils containing boron compounds in Argentina. Journal of Basic Microbiology, 54(6), 568–577. https://doi.org/10.1002/jobm.201200714.
Oren, A. (2002). Molecular ecology of extremely halophilic Archaea and Bacteria. FEMS Microbiology Ecology, 39, 1–7.
Oren, A. (2008). Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Systems, 4(1), 2. https://doi.org/10.1186/1746-1448-4-2.
Oren, A. (2010). Industrial and environmental applications of halophilic microorganisms. Environmental Technology, 31(8–9), 825–834. https://doi.org/10.1080/09593330903370026.
Polti, M. A., Amoroso, M. J., & Abate, C. M. (2007). Chromium (VI) resistance and removal by actinomycete strains isolated from sediments. Chemosphere, 67(4), 660–667.
Pospiech, A., & Neumann, B. (1995). A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends in Genetics, 11(6), 217–218. https://doi.org/10.1016/S0168-9525(00)89052-6.
Rengasamy, P., & Olsson, K. A. (1991). Sodicity and soil structure. Australian Journal of Soil Research, 29(6), 935–952.
Rodriguez-Valera, F. (1988). Characteristics and microbial ecology of hypersaline environments. In F. Rodriguez-Valera (Ed.), Halophilic bacteria (pp. 3–30). Boca Raton: CRC Press.
Sarafin, Y., Donio, M. B. S., Velmurugan, S., Michaelbabu, M., & Citarasu, T. (2014). Kocuria marina BS-15 a biosurfactant producing halophilic bacteria isolated from solar salt works. Saudi Journal of Biological Sciences. https://doi.org/10.1016/j.sjbs.2014.01.001.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680.
Tschitschko, B., Williams, T. J., Allen, M. A., Zhong, L., Raftery, M. J., & Cavicchioli, R. (2016). Ecophysiological distinctions of haloarchaea from a hypersaline Antarctic lake as determined by metaproteomics. Applied and Environmental Microbiology, 82(11), 3165–3173. https://doi.org/10.1128/AEM.00473-16.
Tsuruta, T. (2005). Removal and recovery of lithium using various microorganisms. Journal of Bioscience and Bioengineering, 100(5), 562–566. https://doi.org/10.1263/jbb.100.562.
Ventosa, A. (2006). Unusual micro-organisms from unusual habitats: hypersaline environments. Symposia-society for general microbiology (Vol. 66, pp. 223–255) Cambridge: Cambridge University Press; 1999.
Wong, V. N. L., Greene, R. S. B., Dalal, R. C., & Murphy, B. W. (2010). Soil carbon dynamics in saline and sodic soils: A review. Soil Use and Management, 26(1), 2–11. https://doi.org/10.1111/j.1475-2743.2009.00251.x.
Yumoto, I., Hirota, K., Goto, T., Nodasaka, Y., & Nakajima, K. (2005). Bacillus oshimensis sp. nov., a moderately halophilic, non-motile alkaliphile. International Journal of Systematic and Evolutionary Microbiology, 55, 907–911.
Yumoto, I., Yamaga, S., Sogabe, Y., Nodasaka, Y., Matsuyama, H., Nakajima, K., et al. (2003). Bacillus krulwichiae sp. nov., a halotolerant obligate alkaliphile that utilizes benzoate and m-hydroxybenzoate. International Journal of Systematic and Evolutionary Microbiology, 53, 1531–1536.
Acknowledgements
This project was partially supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 2013-0932), by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 332), and by the Consejo de Investigaciones de la Universidad Nacional de Salta (Salta, Argentina) through the research projects N° 2070/4 and program 2070. Fabiana Lilian Martinez was a recipient of a doctoral fellowship from CONICET.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Martínez, F.L., Orce, I.G., Rajal, V.B. et al. Salar del Hombre Muerto, source of lithium-tolerant bacteria. Environ Geochem Health 41, 529–543 (2019). https://doi.org/10.1007/s10653-018-0148-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0148-2