Abstract
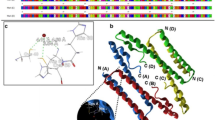
The copper specific binding metallothionein (CuMT) is a type of cysteine-rich, metal-binding, small protein which plays an important role in Cu2+ metabolism in vertebrates. In this study, we investigated the metal tolerance and removing ability of recombinant strains harboring CuMT obtained in vivo from the freshwater crab Sinopotamon henanense (ShCuMT) in order to study its physiological functions and metal binding capacity. We performed a 3D modeling of ShCuMT and created its structural and functional models using the I‐TASSER program. The shCumt gene was inserted into a pGEX-4t-1 vector and recombinant soluble ShCuMT was expressed in Escherichia coli. In addition, in order to characterize the tolerance and removing ability of heavy metals in E. coli with ShCuMT expression, the recombinant strains harboring ShCuMT were exposed to various concentrations of Cd2+, Cu2+ and Zn2+, respectively. The results showed that ShCuMT contains transition metal binding sites. In addition, E. coli cells expressing ShCuMT exhibited enhanced metal tolerance and higher removing ability of metal ions than control cells. However, compared with Cd2+ and Zn2+, E. coli cells expressing ShCuMT have stronger tolerance and higher removing ability of Cu2+. In general, ShCuMT contains multiple transition metal binding sites, and it could enhance tolerance and removing ability of metal ions. Therefore, ShCuMT can provide potential candidates for heavy metal bioremediation. This research on the metal binding properties of ShCuMT provides a scientific basis for bioremediation of heavy metal pollution by the recombinant strains.

Highlights
-
ShCuMT recombinant strain expression enhanced Cd2+, Cu2+ and Zn2+ tolerance.
-
ShCuMT recombinant strain expression enhanced Cd2+, Cu2+ and Zn2+ resistance.
-
ShCuMT recombinant strain expression enhanced Cd2+, Cu2+ and Zn2+ removing ability.
-
ShCuMT can be used as a regulatory biomolecule for Cu2+ homeostasis in Sinopotamon henanense.






Similar content being viewed by others
Data and material availability
All authors ensure that all data and materials support our published claims and comply with field standards.
References
Amiard JC, Amiard-Triquet C, Barka C (2006) Metallothioneins in aquatic invertebrate: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76:160–202. https://doi.org/10.1016/j.aquatox.2005.08.015
Ariöz C, Wittung-Stafshede P (2018) Folding of copper proteins: role of the metal? Q Rev Biophys 51:e4. https://doi.org/10.1017/S0033583518000021
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14:94. https://doi.org/10.3390/ijerph14010094
Bell SG, Vallee BL (2009) The metallothionein/thionein system: an oxidoreductive metabolic zinc link. Chembiochem 10:55–62. https://doi.org/10.1002/cbic.200800511
Beltramini M, Lerch K, Vasak M (1984) Metal substitution of Neurospora copper metallothionein. Biochemistry 23:3422–3427. https://doi.org/10.1021/bi00310a007
Butt TR, Sternberg EJ, Gorman JA, Clark P, Hamer D, Rosenberg M, Crooke ST (1984) Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci USA 81:3332–3336. https://doi.org/10.1073/pnas.81.11.3332
Calderone V, Dolderer B, Hartmann HJ, Echner H, Luchinat C, Bianco CD, Mangani S, Weser U (2005) The crystal structure of yeast copper thionein: the solution of a long-lasting enigma. Proc Natl Acad Sci USA 102:51–56. https://doi.org/10.1073/pnas.0408254101
Calvo J, Jung H, Meloni G (2017) Copper metallothioneins. IUBMB Life 69:236–245. https://doi.org/10.1002/iub.1618
Capdevila M, Bofill R, Palacios O, Atrian S (2012) State-of-the-art of metallothioneins at the beginning of the 21st century. Coord Chem Rev 256:46–62. https://doi.org/10.1016/j.ccr.2011.07.006
Chabicovsky M, Niederstaetter H, Thaler R, Hödl E, Parson W, Rossmanith W, Dallinger R (2003) Localization and quantification of Cd- and Cu-specific metallothionein isoform mRNA in cells and organs of the terrestrial gastropod Helix pomatia. Toxicol Appl Pharmacol 190:25–36. https://doi.org/10.1016/s0041-008x(03)00148-0
Chabicovsky M, Klepal W, Dallinger R (2004) Mechanisms of cadmium toxicity in terrestrial pulmonates: programmed cell death and metallothionein overload. Environ Toxicol Chem 23:648–655. https://doi.org/10.1897/02-617
de Alencar FLS, Navoni JA, do Amaral AS (2017) The use of bacterial bioremediation of metals in aquatic environments in the twenty-first century: a systematic review. Environ Sci Pollut Res Int 24:16545–16559. https://doi.org/10.1007/s11356-017-9129-8
Deng X, Yi XE, Liu G (2007) Cadmium removal from aqueous solution by gene-modified Escherichia coli JM109. J Hazard Mater 139:340–344. https://doi.org/10.1016/j.jhazmat.2006.06.043
Domenech J, Palacios O, Villarreal L, González-Duarte P, Capdevila M, Atrian S (2003) MTO: the second member of a Drosophila dual copper-thionein system. Febs Lett 533:72–78. https://doi.org/10.1016/s0014-5793(02)03754-7
Egli D, Domenech J, Selvaraj A, Balamurugan K, Hua H, Capdevila M, Georgiev O, Schaffner W, Atrian S (2006) The four members of the Drosophila metallothionein family exhibit distinct yet overlapping roles in heavy metal homeostasis and detoxification. Genes Cells 11:647–658. https://doi.org/10.1111/j.1365-2443.2006.00971.x
Egli D, Yepiskoposyan H, Selvaraj A, Balamurugan K, Rajaram R, Simons A, Multhaup G, Mettler S, Vardanyan A, Georgiev O, Schaffner W (2006) A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification. Mol Cell Biol 26:2286–2296. https://doi.org/10.1128/MCB.26.6.2286-2296.2006
Espart A, Marín M, Gil-Moreno S, Palacios Ò, Amaro F, Martín-González A, Gutiérrez JC, Capdevila M, Atrian S (2015) Hints for metal-preference protein sequence determinants: different metal binding features of the five tetrahymena thermophila metallothioneins. Int J Biol Sci 11:456–471. https://doi.org/10.7150/ijbs.11060
Furst P, Hu S, Hackett R, Hamer D (1988) Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell 55:705–717. https://doi.org/10.1016/0092-8674(88)90229-2
Gehrig PM, Dallinger R, You C, Gruber C, Brouwer M, Kagi JHR, Hunziker PE (2000) Electrospray ionization mass spectrometry of zinc, cadmium, and copper metallothioneins: evidence for metal-binding cooperativity. Protein Sci 9:395–402. https://doi.org/10.1110/ps.9.2.395
Gold B, Deng H, Vargas D, Bryk R, Eliezer D, Roberts J, Jiang XJ, Nathan C (2008) Identification of a copper-binding metallothionein in pathogenic Mycobacteria. Nat Chem Biol 4:609–616. https://doi.org/10.1038/nchembio.109
Gupta A, Joia J, Sood A, Sood R, Sidhu C, Kaur G (2016) Microbes as potential tool for remediation of heavy metals: a review. J Microb Biochem Technol 8:364–372. https://doi.org/10.4172/1948-5948.1000310
Hagerman L (1983) Haemocyanin concentration of juvenile lobsters (Homarus gammarus) in relation to moulting cycle and feeding conditions. Mar Biol 77:11–17. https://doi.org/10.1007/BF00393205
Hassoun EA, Stohs SJ (1996) Cd-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J77A.1 cell cultures. Toxicology 112:219–226. https://doi.org/10.1016/0300-483x(96)03404-x
Henkel G, Krebs B (2004) Metallothioneins: zinc, cadmium, mercury, and copper thiolates and selenolates mimicking protein active site features-structural aspects and biological implications. Chem Rev 104:801–824. https://doi.org/10.1021/cr020620d
He YJ, Ma WL, Li YJ, Liu JP, Jing WX, Wang L (2014) Expression of metallothionein of freshwater crab (Sinopotamon henanense) in Escherichia coli enhances tolerance and accumulation of zinc, copper and cadmium. Ecotoxicology 23:56–64. https://doi.org/10.1007/s10646-013-1151-0
Höckner M, Stefanon K, Monteiro F, Vaufleury D, Pérez-Rafael S, Palacios O, Capdevila M, Atrian S, Dallinger R (2011) Physiological relevance and contribution to metal balance of specific and non-specific metallothionein isoforms in the garden snail, Cantareus asperses. Biometals 24:1079–1092. https://doi.org/10.1007/s10534-011-9466-x
Jain S, Arnepalli DN (2019) Biominerlisation as a remediation technique: a critical review. Geotech Charact Geoenviron Eng 16:155–162. https://doi.org/10.1007/978-981-13-0899-4_19
Kang YJ (2006) Metallothionein redox cycle and function. Exp Biol Med 231:1459–1467. https://doi.org/10.1177/153537020623100903
Karin M, Najarian R, Haslinger A, Valenzuela P, Welch J, Fogel S (1984) Primary structure and transcription of an amplified genetic locus: the CUP1 locus of yeast. Proc Natl Acad Sci USA 81:337–341. https://doi.org/10.1073/pnas.81.2.337
Kim SK, Lee BS, Wilson DB, Kim EK (2005) Selective cadmium accumulation using recombinant Escherichia coli. J Biosci Bioeng 99:109–114. https://doi.org/10.1263/jbb.99.109
Lerch K, Ammer D, Olafson RW (1982) Crab metallothionein: primary structures of metallothioneins 1 and 2. J Biol Chem 257:2420–2426. 10;257(5):2420-6
Lee E, Jeon H, Kang C, Woo S, Yum S, Kwon Y (2018) Detection of metallothionein in Javanese medaka (Oryzias javanicus), using a scFv-immobilized protein chip. Sensors 18:1069. https://doi.org/10.3390/s18041069
Limaye DA, Shaikh ZA (1999) Cytotoxicity of cadmium and characteristics of its transport in cardiomyocytes. Toxicol Appl Pharmacol 154:59–66. https://doi.org/10.1006/taap.1998.8575
Liu L, Bilal M, Duan XG, Iqbal HMN (2019) Mitigation of environmental pollution by genetically engineered bacteria-current challenges and future perspectives. Sci Total Environ 667:444–454. https://doi.org/10.1016/j.scitotenv.2019.02.390
Lucon TN, Costa AT, Galvão P, Leite MGP (2020) Cadmium behavior in a karst environment hydrological cycle. Environ Sci Pollut Res 27:8965–8979. https://doi.org/10.1007/s11356-020-07894-2
Ma WL, Wang L, He YJ, Yan Y (2008) Tissue-specific cadmium and metallothionein levels in freshwater crab Sinopotamon henanense during acute exposure to waterborne cadmium. Environ Toxicol 23:393–400. https://doi.org/10.1002/tox.20339
Ma WL, Li XF, Wang Q, Ren ZM, M JCC, Wang L (2019) Tandem oligomeric expression of metallothionein enhance heavy metal tolerance and bioaccumulation in Escherichia coli. Ecotoxicol Environ Saf 181:301–307. https://doi.org/10.1016/j.ecoenv.2019.06.022
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30:261–278. https://doi.org/10.1016/j.envint.2003.08.001
Maret W (2008) Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp Gerontol 43:363–369. https://doi.org/10.1016/j.exger.2007.11.005
Marius B, Patrick W, David WE (1986) Copper-metallothioneins in the American Lobster, Hoarus americanus: potential role as Cu(l) donors to apohemocyanin. Environ Health Perspect 65:93–100. https://doi.org/10.1289/ehp.866593
Ogórek M, Gąsior L, Pierzchała O, Daszkiewicz R, Lenartowicz M (2012) Role of copper in the process of spermatogenesis. Postepy Higieny Medyvyny Doswiadczalnej 71:663–683. https://doi.org/10.5604/01.3001.0010.3846
Ohba KI (2018) Transport and toxicity of cadmium. Nihon eiseigaku zasshi 3:269–274. https://doi.org/10.1265/jjh.73.269
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14:1504. https://doi.org/10.3390/ijerph14121504
Palacios O, Atrian S, Capdevila M (2011) Zn- and Cu-thioneins: a functional classification for metallothioneins? J Biol Inorg Chem 16:991–1009. https://doi.org/10.1007/s00775-011-0827-2
Palacios O, Pérez-Rafael S, Pagani A (2014) Cognate and noncognate metal ion coordination in metal-specific metallothioneins: the Helix pomatia system as a model. J Biol Inorg Chem 19:923–935. https://doi.org/10.1007/s00775-014-1127-4
Pedrini-Martha V, Schnegg R, Baurand PE, deVaufleury A, Dallinger R (2017) The physiological role and toxicological significance of the non-metal-selective cadmium/copper-metallothionein isoform differ between embryonic and adult helicid snails. Comp Biochem Physiol C Toxicol Pharmacol 199:38–47. https://doi.org/10.1016/j.cbpc.2017.02.009
Pérez-Rafael S, Monteiro F, Dallinger R, Atrian S, Palacios O, Capdevila M (2014) Cantareus aspersus metallothionein metal binding abilities: the unspecific CaCd/CuMT isoform provides hints about the metal preference determinants in metallothioneins. Biochim Biophys Acta 1844:1694–707. https://doi.org/10.1016/j.bbapap.2014.06.018
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007. https://doi.org/10.1093/nar/29.9.e45
Purać J, Nikolić TV, Kojić D, Ćelić AS, Plavša JJ, Blagojević DP, Petri ET (2019) Identification of a metallothionein gene in honey bee Apis mellifera and its expression profile in response to Cd, Cu and Pb exposure. Mol Ecol 28:731–745. https://doi.org/10.1111/mec.14984
Rachel AS, Thea HB, Marius B (2000) Cloning and sequencing of cDNAs encoding for a novel copper-specific metallothionein and two cadmium-inducible metallothioneins from the blue crab Callinectes sapidus. Comp Biochem Physiol C 125:325–332. https://doi.org/10.1016/s0742-8413(99)00114-0
Robinson NJ (2008) A bacterial copper metallothionein. Nat Chem Biol 4:582–583. https://doi.org/10.1038/nchembio1008-582
Romero-Isart N, Vasak M (2002) Advances in the structure and chemistry of metallothioneins. J Inorg Biochem 88:388–396. https://doi.org/10.1016/s0162-0134(01)00347-6
Rowland JL, Niederweis M (2012) Resistance mechanisms of Mycobacterium tuberculosis against phagosomal copper overload. Tuberculosis 92:202–210. https://doi.org/10.1016/j.tube.2011.12.006
Sauge-Merle S, Lecomte-Pradines C, Carrier P, Cuiné S, DuBowf M (2012) Heavy metal accumulation by recombinant mammalian metallothionein within Escherichia coli protects against elevated metal exposure. Chemosphere 88:918–924. https://doi.org/10.1016/j.chemosphere.2012.04.015
Scheiber I, Dringen R, Mercer JFB (2013) Copper: effects of deficiency and overload. Metal Ions Life Science 13:359–387. https://doi.org/10.1007/978-94-007-7500-8_11
Sekhar K, Priyanka B, Reddy VD, Rao KV (2011) Metallothionein 1 (CcMT1) of pigeonpea (Cajanus cajan L.) confers enhanced tolerance to copper and cadmium in Escherichia coli and Arabidopsis thaliana. Environ Exp Bot 72:131–139. https://doi.org/10.1016/j.envexpbot.2011.02.017
Serra-Batiste M, Cols N, Alcaraz LA, Donaire A, González-Duarte P, Vasák M (2010) The metal-binding properties of the blue crab copper specific CuMT-2: a crustacean metallothionein with two cysteine triplets. J Biol Inorg Chem 15:759–776. https://doi.org/10.1007/s00775-010-0644-z
Singh S, Mulchandani A, Chen W (2008) Highly selective and rapid arsenic removal by metabolically engineered Escherichia coli cells expressing fucus vesiculosus metallothionein. Appl Environ Microbiol74:2924–2927. https://doi.org/10.1128/AEM.02871-07
Siscar R, Koenig S, Torreblanca A, Sole M (2014) The role of metallothionein and selenium in metal detoxification in the liver of deep-sea fish from the NW Mediterranean Sea. Sci Total Environ 466–467:898–905. https://doi.org/10.1016/j.scitotenv.2013.07.081
Syring RA, Brouwer TH, Brouwer M (2000) Cloning and sequencing of cDNAs encoding for a novel copper-specific metallothionein and two cadmium-inducible metallothioneins from the blue crab Callinectes sapidus. Comparative Biochemistry and Physiology. TComp Biochem Physiol C Toxicol Pharmacol 125:325–332. https://doi.org/10.1016/s0742-8413(99)00114-0
Tak HI, Ahmad F, Babalola OO (2013) Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Environ Contam Toxicol 223:33–52. https://doi.org/10.1007/978-1-4614-5577-6_2
Tzirogiannis KN, Panoutsopoulos GI, Demonakou MD, Hereti RI, Alexandropoulou KN, Basayannis AC, My-koniatis MG (2003) Time-course of cadmium-induced acute hepato-toxicity in the rat liver: the role of apoptosis. Organ Toxicity Mech 77:694–701. https://doi.org/10.1007/s00204-003-0499-y
Valls M, Bofill R, Romero‐Isart N, Gonzàlez-Duarte R, Abián J, Carrascal M, Atrian S, Gonzàlez-Duarte P, Capdevila M (2000) Drosophila MTN: a metazoan copper‐thionein related to fungal forms. Febs Lett 467:189–194. https://doi.org/10.1016/s0014-5793(00)01149-2
Waalkes MP, Infante P, Huff J (1994) The scientific fallacy of route specificity of carcinogenesis with particular reference to cadmium. Regul Toxicol Pharmacol 20:119–121. https://doi.org/10.1006/rtph.1994.1040
Wang CY, He SJ, Zou YM, Liu JL, Zhao RX, Yin XN, Zhang HJ, Li YW (2020) Quantitative evaluation of in-situ bioremediation of compound pollution of oil and heavy metal in sediments from the Bohai Sea, China. Mar Pollut Bull 150:110787. https://doi.org/10.1016/j.marpolbul.2019.110787
Xu DM, Yan B, Chen T, Lei C, Li HZ, Xiao XM (2017) Contaminant characteristics and environmental risk assessment of heavy metals in the paddy soils from lead (Pb)-zinc (Zn) mining areas in Guangdong Province, South China. Environ Sci Pollut Res Int 24:24387–24399. https://doi.org/10.1007/s11356-017-0052-9
Yang HZ, Gu WJ, Chen W, Huang JS, Wang L (2019) Metal binding characterization of heterologously expressed metallothionein of the freshwater crab Sinopotamon henanense. Chemosphere 235:926–934. https://doi.org/10.1016/j.chemosphere.2019.06.097
Yang J, Roy A, Zhang Y (2013) Protein-ligand binding site recognition using complementary binding-specific substructure comparison and sequence profile alignment. Bioinformatics 29:2588–2595. https://doi.org/10.1093/bioinformatics/btt447
Yang J, Sun H, Zhang H, Zhou H (2017) Expression, purification of metallothionein genes from freshwater crab (Sinopotamon yangtsekiense) and development of an antimetallothionein ELISA. PLoS ONE 12:e0174482. https://doi.org/10.1371/journal.pone.0174482
Yang HZ, Wang L, He YJ, Jing WX, Ma WL, Chen CM, Wang L (2020) Analysis of spectrometry and thermodynamics of the metallothionein in freshwater crab Sinopotamon henanense for its binding ability with different metals. Chemosphere 246:125670. https://doi.org/10.1016/j.chemosphere.2019.125670
Zahid MT, Shakoori FR, Zulifqar S, Ahmed I, Al-Ghanim K, Mehboob S, Shakoori AR (2016) Molecular characterization of a copper metallothionein gene from a ciliate Tetrahymena farahensis. J Cell Biochem117:1843–1854
Zhang FQ, Wang YS, Sun CC, Lou ZP, Dong JD (2012) A novel metallothionein gene from a mangrove plant Kandelia candel. Ecotoxicology 21:1633–1641. https://doi.org/10.1007/s10646-012-0952-x
Zhu MC, Shen LY, Tam VWY, Liu Z, Shu TH, Luo WZ (2020) A load-carrier perspective examination on the change of ecological environment carrying capacity during urbanization process in China. Sci Total Environ 714:13684. https://doi.org/10.1016/j.scitotenv.2020.136843
Ziller A, Yadav RK, Capdevila M, Reddy MS, Vallon L, Marmeisse R, Atrian S, Palacios Ò, Fraissinet-Tachet L (2017) Metagenomics analysis reveals a new metallothionein family: sequence and metal-binding features of new environmental cysteine-rich proteins. J Inorg Biochem 167:1–11. https://doi.org/10.1016/j.jinorgbio.2016.11.017
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 31672293), Shanxi Scholarship Council of China (No. 2016-1) and Shanxi Key Research and Development Program of China (No. 201703D221008-3). Thanks to Professors LW and CMC for their suggestions on an earlier draft of the manuscript.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LW, HZY, WLM, CMC and LW. The first draft of the manuscript was written by LW and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Yang, H.Z., Ma, W.L. et al. Study on metal binding capacity of the freshwater crab Sinopotamon henanense’s recombinant copper specific binding metallothionein expressed in Escherichia coli. Ecotoxicology 31, 149–160 (2022). https://doi.org/10.1007/s10646-021-02470-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02470-x