Abstract
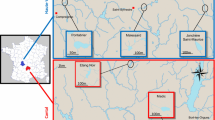
To get closer to the environmental reality, ecotoxicological studies should no longer consider the evaluation of a single pollutant, but rather combination of stress and their interaction. The aim of this study was to determine if responses of a fish to a sudden biological stress could be modified by a prior exposure to a chemical stress (a polymetallic contamination). For this purpose, in situ experiment was conducted in three ponds in the Haute-Vienne department (France). One pond was chosen for its high uranium concentration due to uranium mine tailings, and the two other ponds, which were not submitted to these tailings. Three-spined sticklebacks (Gasterosteus aculeatus) were caged in these ponds for 14 days. After this period, fish were submitted to a biological stress, exerted by lipopolysaccharides injection after anesthesia, and were sacrificed 4 days after these injections for multi-biomarkers analyses (leucocyte viability, phagocytic capacity and reactive oxygen species production, antioxidant peptide and enzymes, lipid peroxidation and DNA damage). The pond which received uranium mine tailings had higher metallic concentrations. Without biological stress, sticklebacks caged in this pond presented an oxidative stress, with increasing of reactive oxygen species levels, modification of some parts of the antioxidant system, and lipid peroxidation. Caging in the two most metal-contaminated ponds resulted in an increase of susceptibility of sticklebacks to the biological stress, preventing their phagocytic responses to lipopolysaccharides and modifying their glutathione contents and glutathione-S-transferase activity.




Similar content being viewed by others
References
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Allen J, Wootton R (1982) The effect of ration and temperature on the growth of the three-spined stickleback, Gasterosteus aculeatus L. J Fish Biol 20:409–422
Allen JRM, Wootton RJ (1984) Temporal patterns in diet and rate of food consumption of the three-spined stickleback (Gasterosteus aculeatus L.) in Llyn Frongoch, an upland Welsh lake. Freshw Biol 14:335–346. doi:10.1111/j.1365-2427.1984.tb00158.x
Almroth BC, Sturve J, Stephensen E, Holth TF, Förlin L (2008) Protein carbonyls and antioxidant defenses in corkwing wrasse (Symphodus melops) from a heavy metal polluted and a PAH polluted site. Mar Environ Res 66:271–277. doi:10.1016/j.marenvres.2008.04.002
Amiard J-C, Amiard Triquet C (2008) Les biomarqueurs dans l’évaluation de l’état écologique des milieux aquatiques. Ed. Tec and Doc, Lavoisier
Andersen F, Lygren B, Maage A, Waagbø R (1998) Interaction between two dietary levels of iron and two forms of ascorbic acid and the effect on growth, antioxidant status and some non-specific immune parameters in Atlantic salmon (Salmo salar) smolts. Aquaculture 161:437–451
Anderson DP (1992) Immunostimulants, adjuvants, and vaccine carriers in fish: applications to aquaculture. Annu Rev Fish Dis 2:281–307
Anderson DP (1996) Environmental factors in fish health: immunological aspects. In: Iwama GK, Nakanishi T (eds) The fish immune system: organism, pathogen, and environment. Academic Press, San Diego, pp 289–310
Babo S, Vasseur P (1992) In vitro effects of Thiram on liver antioxidant enzyme activities of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 22:61–68
Bado-Nilles A, Betoulle S, Geffard A, Porcher JM, Gagnaire B, Sanchez W (2013) Flow cytometry detection of lysosomal presence and lysosomal membrane integrity in the three-spined stickleback (Gasterosteus aculeatus L.) immune cells: applications in environmental aquatic immunotoxicology. Environ Sci Pollut Res Int 20:2692–2704. doi:10.1007/s11356-012-1410-2
Bado-Nilles A, Jolly S, Porcher JM, Palluel O, Geffard A, Gagnaire B, Betoulle S, Sanchez W (2014a) Applications in environmental risk assessment of leucocyte apoptosis, necrosis and respiratory burst analysis on the European bullhead, Cottus sp. Environ Pollut 184:9–17. doi:10.1016/j.envpol.2013.07.049
Bado-Nilles A, Techer R, Porcher JM, Geffard A, Gagnaire B, Betoulle S, Sanchez W (2014b) Detection of immunotoxic effects of estrogenic and androgenic endocrine disrupting compounds using splenic immune cells of the female three-spined stickleback, Gasterosteus aculeatus (L.). Environ Toxicol Pharmacol 38:672–683. doi:10.1016/j.etap.2014.08.002
Barillet S, Buet A, Adam C, Devaux A, Devaux A (2005) Does uranium exposure induce genotoxicity in the teleostean Danio rerio? First experimental results. Radioprotection 40:S175–S181
Béguel JP (2012) Etude de la capacité antioxydante en lien avec la reproduction chez l’huître creuse Crassostrea gigas. Université de Bretagne occidentale, Brest
Bols NC, Brubacher JL, Ganassin RC, Lee LE (2001) Ecotoxicology and innate immunity in fish. Dev Comp Immunol 25:853–873
Boltaña S, Tridico R, Teles M, Mackenzie S, Tort L (2014) Lipopolysaccharides isolated from Aeromonas salmonicida and Vibrio anguillarum show quantitative but not qualitative differences in inflammatory outcome in Sparus aurata (Gilthead seabream). Fish Shellfish Immunol 39:475–482. doi:10.1016/j.fsi.2014.06.003
Bony S, Gillet C, Bouchez A, Margoum C, Devaux A (2008) Genotoxic pressure of vineyard pesticides in fish: field and mesocosm surveys. Aquat Toxicol 89:197–203
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brousseau P, Dunier M, Deschaux P, De Guise S, Krzystyniak K, Fournier M (1997) Marqueurs immunologiques. In: Lagadic L, Caquet TH, Amiard JC, Ramade F (eds) Biomarqueurs en écotoxicologie: aspects fondamentaux. Masson, Paris, p 287–315
Burk RF, Lawrence RA, Correia MA (1980) Sex differences in biochemical manifestations of selenium deficiency in rat liver with special reference to heme metabolism. Biochem Pharmacol 29:39–42
Caldwell CA, Hinshaw J (1994) Physiological and haematological responses in rainbow trout subjected to supplemental dissolved oxygen in fish culture. Aquaculture 126:183–193. doi:10.1016/0044-8486(94)90259-3
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Chilmonczyk S, Monge D (1999) Flow cytometry as a tool for assessment of the fish cellular immune response to pathogens. Fish Shellfish Immunol 9:319–333
Chou H-Y, Peng T-Y, Chang S-J, Hsu Y-L, Wu J-L (1999) Effect of heavy metal stressors and salinity shock on the susceptibility of grouper (Epinephelus sp.) to infectious pancreatic necrosis virus. Virus Res 63:121–129
Cossarini-Dunier M (1987) Effects of the pesticides atrazine and lindane and of manganese ions on cellular immunity of carp, Cyprinus carpio. J Fish Biol 31:67–73. doi:10.1111/j.1095-8649.1987.tb05295.x
Cuenco ML, Stickney RR, Grant WE (1985) Fish bioenergetics and growth in aquaculture ponds: II. Effects of interactions among, size, temperature, dissolved oxygen, unionized ammonia and food on growth of individual fish. Ecol Model 27:191–206. doi:10.1016/0304-3800(85)90002-X
Dautremepuits C, Betoulle S, Vernet G (2002) Antioxidant response modulated by copper in healthy or parasitized carp (Cyprinus carpio L.) by Ptychobothrium sp. (Cestoda). Biochim Biophys Acta 1573:4–8. doi:10.1016/S0304-4165(02)00328-8
De Andrade VM, da Silva J, da Silva FR, Heuser VD, Dias JF, Yoneama ML, de Freitas TRO (2004) Fish as bioindicators to assess the effects of pollution in two southern Brazilian rivers using the Comet assay and micronucleus test. Environ Mol Mutagen 44:459–468. doi:10.1002/em.20070
Dick PT, Dixon DG (1985) Changes in circulating blood cell levels of rainbow trout, Salmo gairdneri Richardson, following acute and chronic exposure to copper. J Fish Biol 26:475–481
Dunier M (1996) Water pollution and immunosuppression of freshwater fish. Ital J Zool 63:303–309
Durai P, Batool M, Choi S (2015) Structure and effects of cyanobacterial lipopolysaccharides. Mar drugs 13:4217–4230
El-Boshy M, Taha R (2011) Effects of mercuric chloride on the immunological, hematological, biochemical parameters and diseases resistance of Nile Tilapia challenged with Aeromnas hydrophila. Nat Sci 9:7–15
Esteve C, Alcaide E, Ureña R (2012) The effect of metals on condition and pathologies of European eel (Anguilla anguilla): in situ and laboratory experiments. Aquat Toxicol 109:176–184. doi:10.1016/j.aquatox.2011.10.002
Eyckmans M, Celis N, Horemans N, Blust R, De Boeck G (2011) Exposure to waterborne copper reveals differences in oxidative stress response in three freshwater fish species. Aquat Toxicol 103:112–120. doi:10.1016/j.aquatox.2011.02.010
Farina M, Avila DS, da Rocha JBT, Aschner M (2013) Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int 62:575–594
Fernandez-Davila ML, Razo-Estrada AC, Garcia-Medina S, Gomez-Olivan LM, Pinon-Lopez MJ, Ibarra RG, Galar-Martinez M (2012) Aluminum-induced oxidative stress and neurotoxicity in grass carp (Cyprinidae-Ctenopharingodon idella). Ecotoxicol Environ Saf 76:87–92. doi:10.1016/j.ecoenv.2011.09.012
Ferreira M, Caetano M, Costa J, Pousao-Ferreira P, Vale C, Reis-Henriques MA (2008) Metal accumulation and oxidative stress responses in, cultured and wild, white seabream from Northwest Atlantic. Sci Total Environ 407:638–646. doi:10.1016/j.scitotenv.2008.07.058
Förstner U, Wittmann GTW (2012) Metal pollution in the aquatic environment. Springer Science and Business Media, New York
Gagnaire B, Bado-Nilles A, Sanchez W (2014) Depleted uranium disturbs immune parameters in zebrafish Danio rerio: an ex vivo/in vivo experiment. Arch Environ Contam Toxicol 67:426–435. doi:10.1007/s00244-014-0022-x
Gagnaire B, Bado-Nilles A, Betoulle S, Amara R, Camilleri V, Cavalié I, Chadili E, Delahaut L, Kerambrun E, Orjollet D, Palluel O, Sanchez W (2015) Former uranium mine-induced effects in caged roach: a multiparametric approach for the evaluation of in situ metal toxicity. Ecotoxicology 24:215–231. doi:10.1007/s10646-014-1374-8
Garcia-Medina S, Razo-Estrada C, Galar-Martinez M, Cortez-Barberena E, Gomez-Olivan LM, Alvarez-Gonzalez I, Madrigal-Bujaidar E (2011) Genotoxic and cytotoxic effects induced by aluminum in the lymphocytes of the common carp (Cyprinus carpio). Comp biochem physiol Toxicol pharmacol 153:113–118. doi:10.1016/j.cbpc.2010.09.005
GEP (2010) Recommandations pour la gestion des anciens sites miniers d’uranium en France. Des sites du Limousin aux autres sites du court aux moyen et long termes. http://www.gep-nucleaire.org/gep/sections/travauxgep/rapports/rapport_final_du_gep/downloadFile/file/RapportGEP-Misenligne_17.09.10.pdf. Accessed Sept 2010
Gill TS, Bianchi CP, Epple A (1992) Trace metal (Cu and Zn) adaptation of organ systems of the American eel, Anguilla rostrata, to external concentrations of cadmium. Comp Biochem Physiol Part C 102:361–371
Grap Limousin (2007) Mesures des pesticides en Limousin. Bilan du suivi 2007. http://www.limousin.developpement-durable.gouv.fr/IMG/pdf/Presentation_des_mesures_cle59111b.pdf. Accessed 27 June 2007
Gust M, Fortier M, Garric J, Fournier M, Gagne F (2013) Immunotoxicity of surface waters contaminated by municipal effluents to the snail Lymnaea stagnalis. Aquat Toxicol 126:393–403. doi:10.1016/j.aquatox.2012.09.001
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hang BT, Milla S, Gillardin V, Phuong NT, Kestemont P (2013) In vivo effects of Escherichia coli lipopolysaccharide on regulation of immune response and protein expression in striped catfish (Pangasianodon hypophthalmus). Fish Shellfish Immunol 34:339–347. doi:10.1016/j.fsi.2012.11.025
Hardie LJ, Fletcher TC, Secombes CJ (1994) Effect of temperature on macrophage activation and the production of macrophage activating factor by rainbow trout (Oncorhynchus mykiss) leucocytes. Dev Comp Immunol 18:57–66
Heier LS, Teien HC, Oughton D, Tollefsen KE, Olsvik PA, Rosseland BO, Lind OC, Farmen E, Skipperud L, Salbu B (2013) Sublethal effects in Atlantic salmon (Salmo salar) exposed to mixtures of copper, aluminium and gamma radiation. J Environ Radioact 121:33–42. doi:10.1016/j.jenvrad.2012.04.004
Herlory O, Bonzom JM, Gilbin R, Frelon S, Fayolle S, Delmas F, Coste M (2013) Use of diatom assemblages as biomonitor of the impact of treated uranium mining effluent discharge on a stream: case study of the Ritord watershed (Center-West France). Ecotoxicology 22:1186–1199. doi:10.1007/s10646-013-1106-5
Hetrick FM, Knittel MD, Fryer JL (1979) Increased susceptibility of rainbow trout to infectious hematopoietic necrosis virus after exposure to copper. Appl Environ Microbiol 37:198–201
Hynes H (1950) The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. J Anim Ecol 19:36–58
Ingram GA (1980) Substances involved in the natural resistance of fish to infection: A review. J Fish Biol 16:23–60. doi:10.1111/j.1095-8649.1980.tb03685.x
IRSN (2007) Inventaire national des sites miniers d’uranium. http://www.irsn.fr/FR/connaissances/Environnement/expertises-locales/sites-miniers-uranium/Documents/irsn_mines-uranium_mimausa_notice_explicative.pdf. Accessed 2 Sept 2007
Jiang J, Shi D, Zhou X-Q, Hu Y, Feng L, Liu Y, Jiang W-D, Zhao Y (2015) In vitro and in vivo protective effect of arginine against lipopolysaccharide induced inflammatory response in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 42:457–464. doi:10.1016/j.fsi.2014.11.030
Jolly S, Jaffal A, Delahaut L, Palluel O, Porcher J-M, Geffard A, Sanchez W, Betoulle S (2014) Effects of aluminium and bacterial lipopolysaccharide on oxidative stress and immune parameters in roach, Rutilus rutilus L. Environ Sci Pollut Res 21:13103–13117
Kelly JM, Janz DM (2009) Assessment of oxidative stress and histopathology in juvenile northern pike (Esox lucius) inhabiting lakes downstream of a uranium mill. Aquat Toxicol 92:240–249. doi:10.1016/j.aquatox.2009.02.007
Kerambrun E, Henry F, Perrichon P, Courcot L, Meziane T, Spilmont N, Amara R (2012) Growth and condition indices of juvenile turbot Scophthalmus maximus, exposed to contaminated sediments: effects of metallic and organic compounds. Aquat Toxicol 108:130–140. doi:10.1016/j.aquatox.2011.07.016
Knapp S, de Vos AF, Florquin S, Golenbock DT, van der Poll T (2003) Lipopolysaccharide binding protein is an essential component of the innate immune response to Escherichia coli peritonitis in mice. Infect Immun 71:6747–6753
Knittel M (1981) Susceptibility of steelhead trout Salmo gairdneri Richardson to redmouth infection Yersinia ruckeri following exposure to copper. J Fish Dis 4:33–40
Kumar PA, Rajagopal G (2003) Lipid peroxidation in erythrocytes of patients with type 2 diabetes mellitus. Indian J Clin Biochem 18:71–74
Laroche J, Quiniou L, Juhel G, Auffret M, Moraga D (2002) Genetic and physiological responses of flounder (Platichthys flesus) populations to chemical contamination in estuaries. Environ Toxicol Chem 21:2705–2712
Lawrence DA (1981) Heavy metal modulation of lymphocyte activities: I. In vitro effects of heavy metals on primary humoral immune responses. Toxicol appl pharmacol 57:439–451
Le Guernic A, Sanchez W, Bado-Nilles A, Palluel O, Turies C, Chadili E, Cavalié I, Delahaut L, Adam-Guillermin C, Porcher J-M, Geffard A, Betoulle S, Gagnaire B (2016) In situ effects of metal contamination from former uranium mining sites on the health of the three-spined stickleback (Gasterosteus aculeatus, L.). Ecotoxicology 25:1234–1259. doi:10.1007/s10646-016-1677-z
Ling KH, Sin YM, Lam TJ (1993) Effect of copper sulphate on ichthyophthiriasis (white spot disease) in goldfish (Carassius auratus). Aquaculture 118:23–35. doi:10.1016/0044-8486(93)90277-6
Lorin-Nebel C, Felten V, Blondeau-Bidet E, Grousset E, Amilhat E, Simon G, Biagianti S, Charmantier G (2013) Individual and combined effects of copper and parasitism on osmoregulation in the European eel Anguilla anguilla. Aquat toxicol 130–131:41–50. doi:10.1016/j.aquatox.2012.11.018
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Maceda-Veiga A, Monroy M, Navarro E, Viscor G, de Sostoa A (2013) Metal concentrations and pathological responses of wild native fish exposed to sewage discharge in a Mediterranean river. Sci Total Environ 449:9–19. doi:10.1016/j.scitotenv.2013.01.012
MacFarlane RD, Bullock GL, McLaughlin JJA (1986) Effects of five metals on susceptibility of striped bass to Flexibacter columnaris. Trans Am Fish Soc 115:227–231
Marques SM, Chaves S, Goncalves F, Pereira R (2013) Evaluation of growth, biochemical and bioaccumulation parameters in Pelophylax perezi tadpoles, following an in situ acute exposure to three different effluent ponds from a uranium mine. Sci Total Environ 445–446:321–328. doi:10.1016/j.scitotenv.2012.12.080
Mitchelmore C, Chipman J (1998) DNA strand breakage in aquatic organisms and the potential value of the comet assay in environmental monitoring. Mutat Res Fundam Mol Mech of Mutagen 399:135–147
Moore MN, Lowe D, Köhler A (2004) Biological effects of contaminants: measurement of lysosomal membrane stability. International Council for the Exploration of the Sea, vol. 36
Münzing J (1963) The evolution of variation and distributional patterns in European populations of the three-spined stickleback, Gasterosteus aculeatus. Evolution 17:320–332
Ndong D, Chen Y-Y, Lin Y-H, Vaseeharan B, Chen J-C (2007) The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol 22:686–694
OECD (2000) Eleventh addendum to the OECD guidelines for testing of chemicals. OECD Publishing, Paris
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oliva M, Jose Vicente J, Gravato C, Guilhermino L, Dolores Galindo-Riano M (2012) Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a Huelva estuary (SW Spain): seasonal and spatial variation. Ecotoxicol Environ Saf 75:151–162. doi:10.1016/j.ecoenv.2011.08.017
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pandey S, Parvez S, Ansari RA, Ali M, Kaur M, Hayat F, Ahmad F, Raisuddin S (2008) Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish Channa punctata Bloch. Chem Biol Interact 174:183–192. doi:10.1016/j.cbi.2008.05.014
Paoletti F, Aldinucci D, Mocali A, Caparrini A (1986) A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem 154:536–541
Park BS, Song DH, Kim HM, Choi B-S, Lee H, Lee J-O (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195
Pereira R, Antunes SC, Marques SM, Goncalves F (2008) Contribution for tier 1 of the ecological risk assessment of Cunha Baixa uranium mine (Central Portugal): I soil chemical characterization. Sci Total Environ 390:377–386. doi:10.1016/j.scitotenv.2007.08.051
Pijanowski L, Scheer M, Verburg-van Kemenade BML, Chadzinska M (2015) Production of inflammatory mediators and extracellular traps by carp macrophages and neutrophils in response to lipopolysaccharide and/or interferon-y2. Fish Shellfish Immunol 42:473–482. doi:10.1016/j.fsi.2014.11.019
Playle RC (1998) Modelling metal interactions at fish gills. Sci Total Environ 219:147–163
Pottinger T, Carrick T, Yeomans W (2002) The three-spined stickleback as an environmental sentinel: effects of stressors on whole-body physiological indices. J Fish Biol 61:207–229
Qu R, Feng M, Wang X, Qin L, Wang C, Wang Z, Wang L (2014) Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat Toxicol 150:9–16. doi:10.1016/j.aquatox.2014.02.008
Rachmawati D, Bontkes HJ, Verstege MI, Muris J, von Blomberg BME, Scheper RJ, van Hoogstraten IMW (2013) Transition metal sensing by Toll-like receptor-4: next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermat 68:331–338
Raghavan B, Martin SF, Esser PR, Goebeler M, Schmidt M (2012) Metal allergens nickel and cobalt facilitate TLR4 homodimerization independently of MD2. EMBO Rep 13:1109–1115. doi:10.1038/embor.2012.155
Rebl A, Goldammer T, Seyfert H-M (2010) Toll-like receptor signaling in bony fish. Vet Immunol Immunopathol 134:139–150
Regoli F, Nigro M, Orlando E (1998) Lysosomal and antioxidant responses to metals in the Antarctic scallop Adamussium colbecki. Aquat Toxicol 40:375–392. doi:10.1016/S0166-445X(97)00059-3
Requintina PJ, Oxenkrug GF (2003) Differential effects of lipopolysaccharide on lipid peroxidation in F344N, SHR rats and BALB/c mice, and protection of melatonin and NAS against its toxicity. Ann N Y Acad Sci 993:325–333
Roales RR, Perlmutter A (1977) The effects of sub-lethal doses of methylmercury and copper, applied singly and jointly, on the immune response of the blue gourami (Trichogaster trichopterus) to viral and bacterial antigens. Arch Environ Contam Toxicol 5:325–331. doi:10.1007/bf02220914
Robohm RA (1986) Paradoxical effects of cadmium exposure on antibacterial antibody responses in two fish species: inhibition in cunners (Tautogolabrus adspersus) and enhancement in striped bass (Morone saxatilis). Vet Immunol Immunopathol 12:251–262. doi:10.1016/0165-2427(86)90129-7
Rosenfeld Y, Shai Y (2006) Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions: role in bacterial resistance and prevention of sepsis. Biochim Biophys Acta (BBA)-Biomembr 1758:1513–1522
Rougier F, Menudier A, Bosgiraud C, Nicolas J (1996) Copper and zinc exposure of zebrafish, Brachydanio rerio (Hamilton–Buchaman): effects in experimental listeria infection. Ecotoxicol Environ Saf 34:134–140
Roussel H, Joachim S, Lamothe S, Palluel O, Gauthier L, Bonzom JM (2007) A long-term copper exposure on freshwater ecosystem using lotic mesocosms: individual and population responses of three-spined sticklebacks (Gasterosteus aculeatus). Aquat Toxicol 82:272–280. doi:10.1016/j.aquatox.2007.02.018
Ruas CB, dos Carvalho SC, de Araujo HS, Espindola EL, Fernandes MN (2008) Oxidative stress biomarkers of exposure in the blood of cichlid species from a metal-contaminated river. Ecotoxicol Environ Saf 71:86–93. doi:10.1016/j.ecoenv.2007.08.018
Sanchez W, Palluel O, Meunier L, Coquery M, Porcher JM, Ait-Aissa S (2005) Copper-induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Environ Toxicol Pharmacol 19:177–183. doi:10.1016/j.etap.2004.07.003
Sanchez W, Ait-Aissa S, Palluel O, Ditche JM, Porcher JM (2007) Preliminary investigation of multi-biomarker responses in three-spined stickleback (Gasterosteus aculeatus L.) sampled in contaminated streams. Ecotoxicology 16:279–287. doi:10.1007/s10646-006-0131-z
Sanchez W, Katsiadaki I, Piccini B, Ditche JM, Porcher JM (2008a) Biomarker responses in wild three-spined stickleback (Gasterosteus aculeatus L.) as a useful tool for freshwater biomonitoring: a multiparametric approach. Environ Int 34:490–498. doi:10.1016/j.envint.2007.11.003
Sanchez W, Piccini B, Ditche JM, Porcher JM (2008b) Assessment of seasonal variability of biomarkers in three-spined stickleback (Gasterosteus aculeatus L.) from a low contaminated stream: implication for environmental biomonitoring. Environ Int 34:791–798. doi:10.1016/j.envint.2008.01.005
Sanchez-Dardon J, Voccia I, Hontela A, Chilmonczyk S, Dunier M, Boermans H, Blakley B, Fournier M (1999) Immunomodulation by heavy metals tested individually or in mixtures in rainbow trout (Oncorhynchus mykiss) exposed in vivo. Environ Toxicol Chem 18:1492–1497
Santos R, Palos-Ladeiro M, Besnard A, Porcher JM, Bony S, Sanchez W, Devaux A (2013) Relationship between DNA damage in sperm after ex vivo exposure and abnormal embryo development in the progeny of the three-spined stickleback. Reprod Toxicol 36:6–11. doi:10.1016/j.reprotox.2012.11.004
Saxena M, Gopal K, Jones W, Ray P (1992) Immune responses to Aeromonas hydrophila in cat fish (Heteropneustis fossilis) exposed to cadmium and hexachlorocyclohexane. Bull Environ Contam Toxicol 48:194–201
Sepulcre MP, Alcaraz-Pérez F, López-Muñoz A, Roca FJ, Meseguer J, Cayuela ML, Mulero V (2009) Evolution of lipopolysaccharide (LPS) recognition and signaling: fish TLR4 does not recognize LPS and negatively regulates NF-κB activation. J Immunol 182:1836–1845
Sevcikova M, Modra H, Slaninova A, Svobodova Z (2011) Metals as a cause of oxidative stress in fish: a review. Vet Med 56:537–546
Sewerynek E, Melchiorri D, Reiter RJ, Ortiz GG, Lewinski A (1995) Lipopolysaccharide-induced hepatotoxicity is inhibited by the antioxidant melatonin. Eur J Pharmacol Environ Toxicol Pharmacol 293:327–334. doi:10.1016/0926-6917(95)90052-7
Sheir SK, Handy RD (2010) Tissue injury and cellular immune responses to cadmium chloride exposure in the common mussel Mytilus edulis: modulation by lipopolysaccharide. Arch Environ Contam Toxicol 59:602–613. doi:10.1007/s00244-010-9502-9
Sindayigaya E, Van Cauwenbergh R, Robberecht H, Deelstra H (1994) Copper, zinc, manganese, iron, lead, cadmium, mercury and arsenic in fish from Lake Tanganyika Burundi. Sci Total Environ 144:103–115
Spry DJ, Wiener JG (1991) Metal bioavailability and toxicity to fish in low-alkalinity lakes: a critical review. Environ Pollut 71:243–304. doi:10.1016/0269-7491(91)90034-T
Stadtman E, Oliver C (1991) Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem 266:2005–2008
Stolen JS, Fletcher TC (1994) Modulators of Fish Immune Responses. In: Stolen JS (ed) Models for environmental toxicology/biomarkers immunostimulators, vol 1. SOS Publications, Fair Haven
Sugino K, Dohi K, Yamada K, Kawasaki T (1987) The role of lipid peroxidation in endotoxin-induced hepatic damage and the protective effect of antioxidants. Surgery 101:746–752
Swain P, Nayak SK, Nanda PK, Dash S (2008) Biological effects of bacterial lipopolysaccharide (endotoxin) in fish: a review. Fish Shellfish Immunol 25:191–201. doi:10.1016/j.fsi.2008.04.009
Vandeputte C, Guizon I, Genestie-Denis I, Vannier B, Lorenzon G (1994) A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: performance study of a new miniaturized protocol. Cell Biol Toxicol 10:415–421
Vieira MC, Torronteras R, Cordoba F, Canalejo A (2012) Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicol Environ Saf 78:212–217. doi:10.1016/j.ecoenv.2011.11.015
WaagbØ R (1994) The impact of nutritional factors on the immune system in Atlantic salmon, Salmo salar L.: a review. Aquac Res 25:175–197. doi:10.1111/j.1365-2109.1994.tb00573.x
Wirzinger G, Weltje L, Gercken J, Sordyl H (2007) Genotoxic damage in field-collected three-spined sticklebacks (Gasterosteus aculeatus L.): a suitable biomonitoring tool? Mutat Res 628:19–30. doi:10.1016/j.mrgentox.2006.11.011
Wootton RJ (1984) A functional biology of sticklebacks. Univ of California Press, Berkeley
Xiang L-X, Peng B, Dong W-R, Yang Z-F, Shao J-Z (2008) Lipopolysaccharide induces apoptosis in Carassius auratus lymphocytes, a possible role in pathogenesis of bacterial infection in fish. Dev Comp Immunol 32:992–1001
Yadav KK, Trivedi SP (2009) Sublethal exposure of heavy metals induces micronuclei in fish Channa punctata. Chemosphere 77:1495–1500. doi:10.1016/j.chemosphere.2009.10.022
Zelikoff JT (1993) Metal pollution-induced immunomodulation in fish. Annu Rev Fish Dis 3:305–325
Acknowledgments
The authors are deeply grateful to the owners of the different ponds for having allowed this experiment. S. Pierrisnard, D. Orjollet, V. Camilleri, L. Carasco and S. Frelon are acknowledged for their help with laboratory analyses. This work was partly supported by IRSN, the financial support of the 181 DRC 46 program of the French Ministry for Ecology and Sustainable Development, and the ECCOREV research federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
These experiments were conducted in accordance with the European Commission’s recommendation 2007/526/EC on revised guidelines for the accommodation and care of animals used for experimental and other scientific purposes. The registration number for ethics of INERIS laboratory is the B60-769-02. For sampling, to avoid bias in the immune responses, no anaesthetic overdose can be used for euthanasia of sticklebacks. Indeed, in the 2010/63/EU Directive of the European Parliament and the 22 September 2010 Council about the protection of animals used for scientific purposes, this option is envisaged “if anesthesia is incompatible with the purpose of the procedure”. Therefore, sticklebacks were rapidly sacrificed by cervical dislocation followed by destruction of the brain. This method has been approved by the Committee No. 96-CREMEAP (Regional Ethics Committee in Animal Experimentation of Picardy).
Rights and permissions
About this article
Cite this article
Le Guernic, A., Sanchez, W., Palluel, O. et al. Acclimation capacity of the three-spined stickleback (Gasterosteus aculeatus, L.) to a sudden biological stress following a polymetallic exposure. Ecotoxicology 25, 1478–1499 (2016). https://doi.org/10.1007/s10646-016-1699-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-016-1699-6