Abstract
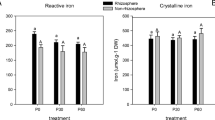
Metal pollution has been widely reported in mangrove wetlands; however, the mechanisms involved in metal detoxification by mangroves are still poorly understood. This study aimed to investigate the possible function of root lignification/suberization on Pb uptake and tolerance in mangroves. Two mangroves, Acanthus ilicifolius and Rhizophora stylosa with different root lignification/suberization were selected as plant materials; the former exhibits a thin exodermis and low lignification/suberization, while the latter possesses a thick exodermis and high lignification/suberization. A pot trial with addition of Pb was conducted to investigate the differences in Pb uptake and tolerance between the two mangroves. The experiment of rhizobox was designed to explore Pb dynamics and availabilities in the rhizosphere soils, besides, the ability of Pb uptake by the excised roots and X-ray analysis for Pb distribution within roots were also detected. The results revealed that R. stylosa exhibited relatively higher Pb tolerance together with less Pb accumulations when compared to A. ilicifolius. For both species, lower proportion of exchangeable and Carbonate Pb and higher higher Fe–Mn oxides Pb were observed in the rhizosphere zone when compared to the respective non-rhizosphere zone. The results from metal uptake by the excised roots and X-ray analysis clearly showed that the thick lignified/suberized exodermis of R. stylosa could more efficiently delay Pb entering into the roots, leading to less Pb accumulation. In summary, the present study proposes a barrier property of the lignified/suberized exodermis in dealing with the stresses of Pb.






Similar content being viewed by others
References
Agoramoorthy G, Chen FA, Hsu MJ (2008) Threat of heavy metal pollution in halophtytic and mangrove plants of Tamil Nadu, India. Environ Pollut 155:320–326
Alongi DM (2002) Present state and future of the world’s mangrove forests. Environ Conserv 29:331–349
Armstrong J, Armstrong W (2001) Rice and Phragamites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. Am J Bot 88:1359–1370
Armstrong J, Armstrong W (2005) Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Ann Bot 96:625–638
Armstrong W, Beckett PM (1987) Internal aeration and the development of stelar anoxia in submerged roots: a multishelled mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytol 105:221–245
Armstrong W, Wright E (1975) The theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiol Plant 35:21–26
Brundrett MC, Kendrick B, Peterson CA (1991) Efficient lipid staining in plant materials with Sudan red 7B or Fluorol yellow 088 in polyethylene glycol-glycerol. Biotech Histochem 66:111–116
Chen KP, Jiao JJ (2008) Metal concentrations and mobility and mobility in marine and groundwater in coastal reclamation areas: a case study in Shenzhen, China. Environ Pollut 151:576–584
Cheng H, Liu Y, Tam NFY, Wang X, Li SY, Chen GZ, Ye ZH (2010) The role of radial oxygen loss and root anatomy on zinc uptake and tolerance in mangrove seedlings. Environ Pollut 158:1189–1196
Cheng H, Tam NFY, Wang YS, Li SY, Chen GZ, Ye ZH (2012a) Effect of copper on growth, radial oxygen loss and root permeability of seedlings of mangroves Bruguiera gymnorrhiza and Rhizophora stylosa. Plant Soil 359:255–266
Cheng H, Wang YS, Ye ZH, Chen DT, Wang YT, Peng YL, Wang LY (2012b) Influence of N deficiency and salinity on metal (Pb, Zn and Cu) accumulation and tolerance by Rhizophora stylosa in relation to root anatomy and permeability. Environ Pollut 164:110–117
Cheng H, Wang MY, Wong MH, Ye ZH (2014) Does radial oxygen loss and iron plaque formation on roots alter Cd and Pb uptake and distribution in rice plant tissues? Plant Soil 375:137–148
Clark MW, McConchie D, Lewis DW, Saenger P (1998) Redox stratification and heavy metal partitioning in Avicennia-dominated mangrove sediments: a geochemical model. Chem Geol 149:147–171
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Pedersen O (2008) Oxygen dynamics in submerged rice (Oryza sativa). New Phytol 178:326–334
Degenhardt B, Gimmler H (2000) Cell wall adaptations to multiple environmental stresses in maize roots. Plant Cell Environ 51:595–603
Garthwaite AJ, Steudle E, Colmer TD (2006) Water uptake by roots of Hordeum marinum: formation of a barrier to radial O2 loss does not affect root hydraulic conductivity. J Exp Bot 57:655–664
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001) The exodermis: a variable apoplastic barrier. J Exp Bot 52:2245–2264
Huang GY, Wang YS (2010) Physiological and biochemical responses in the leaves of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) exposed to multiple heavy metals. J Hazard Mater 182:848–854
Jackson MB, Armstrong W (1999) Formation of aerenchyma and processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1:274–287
Jacob DL, Otte ML (2003) Conflicting process in the wetland plant rhizosphere: metal retention or mobilization? Water Air Soil Pollut 3:91–104
Krauss KW, Lovelock CE, Mckee KL, Lopez-Hoffman HL, Ewe SML, Sousa WP (2008) Environmental drivers in mangrove establishment and early development: a review. Aquat Bot 89:105–127
Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK (2009) The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planta 230:119–134
Krishnamurthy P, Jyothi-Prakash PA, Qin L, Lin QS, Loh CS, Kumar PP (2014) Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis. Plant Cell Environ 37:1656–1671
Kulichikhin K, Yamauchi T, Watanabe K, Nakazono M (2014) Biochemical and molecular characterization of rice (Oryza sativa L.) roots forming a barrier to radial oxygen loss. Plant Cell Environ 37:2406–2420
MacFarlane GR, Burchett MD (1999) Zinc distribution and excretion in the leavesof grey mangrove, Avicennia marina (Forsk.) Vierh. Environ Exp Bot 41:167–175
MacFarlane GR, Burchett MD (2000) Cellular distribution of copper, lead and zinc in the grey mangrove, Avicennia marina (Forsk.) Vierh. Aquat Bot 68:45–59
MacFarlane GR, Burchett MD (2002) Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove Avicennia marina (Forsk.) Vierh. Mar Environ Res 54:65–84
Mendoza DG, Moreno AQ, Perez OZ (2007) Coordinated responses of phytochelatin synthase and metallothionein genes in black mangrove, Avicennia germinans, exposed to cadmium and copper. Aquat Toxicol 83:306–314
Meyer CJ, Peterson CA, Steudle E (2011) Permeability of lris germanica’s multiseriate exodermis to water, NaCl, and ethanol. J Exp Bot 62:1911–1926
Nath B, Brich G, Chauduri P (2014) Assessment of sediment quality in Avicennia marina-dominated embayments of Sydney Estuary: the potential use of pneumatophores (aerial roots) as a bio-indicator of trace metal contamination. Sci Total Environ 472:1010–1022
Nishizono H (1987) The role of root cell wall in heavy metal tolerance of Athyriun yokoscense. Plant Soil 101:15–21
Otte ML, Rozema J, Koster L, Haarsma MS, Broekman RA (1989) Iron plaque on roots of Aster tripolium L.: interaction with zinc uptake. New Phytol 111:309–317
Peters EC, Gassman NJ, Firman JR, Richmond H, Power EA (1997) Ecotoxicology of tropical marine ecosystems. Environ Toxicol Chem 16:12–40
Pi N, Tam NFY, Wu Y, Wong MH (2009) Root anatomy and spatial pattern of radial oxygen loss of eight true mangrove species. Aquat Bot 90:222–230
Pollard M, Beisson F, Li YH, Ohlrogge JB (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13:236–246
Qiu YW, Yu KF, Zhang G, Wang WX (2011) Accumulation and partitioning of seven trace metals in mangroves and sediment cores from three estuarine wetlands of Hainan Island, China. J Hazard Mater 190:631–638
Schreiber L, Hartmann K, Skrabs M, Zeier J (1999) Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot 50:1267–1280
Schreiber L, Franke R, Hartmann K (2005) Effect of NO3 deficiency and NaCl stress on suberin deposition rhizo-and hypodermal and endodermal cell walls of castor bean (Ricinus communis L.) roots. Plant Soil 269:333–339
Simone OD, Haase K, Müller E, Junk WJ, Hartmann K, Schreiber L, Schmidt W (2003) Apoplasmic barriers and oxygen transport properties of hypodermal cell walls in roots from four Amazonian tree species. Plant Physiol 132:206–217
Song H, Wang YS, Sun CC, Wang YT, Peng YL, Cheng H (2012) Effects of pyrene on antioxidant systems and lipid peroxidation level in mangrove plants, Bruguiera gymnorrhiza. Ecotoxicology 21:1625–1632
Soukup A, Armstrong W, Schreiber L, Franke R, Votrubová O (2007) Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytol 173:264–278
Tam NFY, Wong YS (1997) Accumulation and distribution of heavy metals in a simulated mangrove system treated with sewage. Hydrobiologia 352:67–75
Tam NFY, Wong YS (2000) Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ Pollut 110:195–205
Tam NFY, Li SH, Lan CY, Chen GZ, Li MS, Wong YS (1995) Nutrients and heavy metal contamination of plants and sediments in Futian mangrove forest. Hydrobiologia 295:149–158
Tessier A, Campell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Van de Mortel J, Schat H, Moerland PD, Loren Ver, van Themaat E, Van der Ent S, Blankenstijen H, Ghandilyan A, Tsiatsiani S, Aarts MGM (2008) Expression differences for genes involved in lignin, glutathione and sulphate metabolism in response to cadmium in Arabidopsis thaliana and the related Zn/Cd hyperaccumulator Thlaspi caerulescens. Plant Cell Environ 31:301–324
Yang JX, Liu Y, Ye ZH (2012) Root-induced changes of pH, Eh, Fe(II) and fractions of Pb and Zn in rhizosphere soils of four wetland plants with different radial oxygen loss. Pedosphere 22:518–527
Youssef T, Saenger P (1996) Anatomical adaptive strategies to flooding and rhizosphere oxidation in mangrove seedlings. Aust J Bot 44:297–313
Zeier J, Schreiber L (1998) Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of five monocotyledoneous species: chemical composition in relation to fine structure. Planta 206:349–361
Zeier J, Goll A, Yokoyama M, Karahara I, Schreiber L (1999) Structure and chemical composition of endodermal and rhizodermal/hypodermal walls of several species. Plant Cell Environ 22:271–279
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Zhang FQ, Wang YS, Sun CC, Lou ZP, Dong JD (2012) A novel metallothionein gene from a mangrove plant Kandelia candel. Ecotoxicology 21:1633–1641
Acknowledgments
This research was supported by the key projects in the National Science & Technology Pillar Program in the Eleventh Five-year Plan Period (No. 2012BAC07B0402), the National Natural Science Foundation of China (Nos. 41106103, 41430966, 41176101), Natural Science Foundation of Guangdong province (2014A030313783) and the Fund of Key Laboratory for Exploitation & Utilization of Marine Fisheries Resource in South China Sea, Ministry of Agriculture (LSF2013-5).
Conflict of interest
The authors declare that they have no conflict of interest associated with this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, H., Wang, YS., Liu, Y. et al. Pb uptake and tolerance in the two selected mangroves with different root lignification and suberization. Ecotoxicology 24, 1650–1658 (2015). https://doi.org/10.1007/s10646-015-1473-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1473-1