Abstract
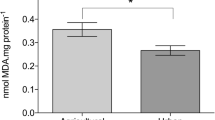
Historical discharges of Hg into the South River near the town of Waynesboro, VA, USA, have resulted in persistently elevated Hg concentrations in sediment, surface water, ground water, soil, and wildlife downstream of the discharge site. In the present study, we examined mercury (Hg) levels in in little brown bats (Myotis lucifugus) from this location and assessed the utility of a non-destructively collected tissue sample (wing punch) for determining mitochondrial DNA (mtDNA) damage in Hg exposed bats. Bats captured 1 and 3 km from the South River, exhibited significantly higher levels of total Hg (THg) in blood and fur than those from the reference location. We compared levels of mtDNA damage using real-time quantitative PCR (qPCR) analysis of two distinct regions of mtDNA. Genotoxicity is among the many known toxic effects of Hg, resulting from direct interactions with DNA or from oxidative damage. Because it lacks many of the protective protein structures and repair mechanisms associated with nuclear DNA, mtDNA is more sensitive to the effects of genotoxic chemicals and therefore may be a useful biomarker in chronically exposed organisms. Significantly higher levels of damage were observed in both regions of mtDNA in bats captured 3 km from the river than in controls. However, levels of mtDNA damage exhibited weak correlations with fur and blood THg levels, suggesting that other factors may play a role in the site-specific differences.







Similar content being viewed by others
References
Baker R, Lavie B, Nevo E (1985) Natural selection for resistance to mercury pollution. Cell Mol Life Sci 41(5):697–699
Belyaeva EA, Sokolova TV, Emelyanova LV, Zakharova IO (2012) Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copper. Sci World J 2012:136063
Ben-Ozer EY, Rosenspire AJ, McCabe MJ Jr, Worth RG, Kindzelskii AL, Warra NS, Petty HR (2000) Mercuric chloride damages cellular DNA by a non-apoptotic mechanism. Mutat Res 470:19–27
Bergeron CM, Bodinof CM, Unrine JM, Hopkins WA (2010) Bioaccumulation and maternal transfer of mercury and selenium in amphibians. Environ Toxicol Chem 29(4):989–997
Cantoni O, Costa M (1983) Correlations of DNA strand breaks and their repair with cell survival following acute exposure to mercury (II) and X-rays. Mol Pharmacol 24:84–87
Cardona-Marek T, Knott KK, Meyer BE, O’Hara TM (2009) Mercury concentrations in Southern Beaufort Sea polar bears: variation based on stable isotopes of carbon and nitrogen. Environ Toxicol Chem 28(7):1416–1424
Carter LJ (1977) Chemical plants leave unexpected legacy for two Virginia rivers. Science 198:1015–1020
Cline SD (2012) Mitochondrial DNA damage and its consequences for mitochondrial gene expression. Biochim Biophys Acta 1819(9/10):979–991
Crespo-Lopez ME, Macedo GL, Pereira SID, Arrifano GPF, Picanco-Diniz DLW, do Nascimento JLM, Herculano AM (2009) Mercury and human genotoxicity: critical considerations and possible molecular mechanisms. Pharmacol Res 60(4):212–220
Crespo-Lópeza ME, Macêdoa GL, Pereirab SID, Arrifanoa GPF, Picanço-Dinizc DLW, Nascimentod JLMD, Herculano AM (2009) Mercury and human genotoxicity: critical considerations and possible molecular mechanisms. Pharmacol Res 60(4):212–220
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger KK, Monroe AP, White AE (2008) The movement of aquatic mercury through Terrestrial food webs. Science 320(5874):335
DelVecchio R, Friedman S, Unsworth R (2010) South river and South Fork of the Shenandoah River natural resource damage assessment: draft damage assessment plan. Industrial Economics Incorporated, Cambridge
Drummond AJ, Ashton B, Cheung M, Heled J, Kearse M, Moir R, Stones-Havas S, Thierer T, Wilson A (2009) Geneious v4.75. http://www.geneious.com
Edwards JG (2009) Quantification of mitochondrial DNA (mtDNA) damage and error rates by real-time QPCR. Mitochondrion 9(1):31–35
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal induced oxidative damage. Curr Top Med Chem 1:529–539
Grotto D, Barcelos GM, Valentini J, Antunes LG, Angeli J, Garcia S, Barbosa F Jr (2009) Low levels of methylmercury induce DNA damage in rats: protective effects of selenium. Arch Toxicol 83(3):249–254
Jung D, Cho Y, Collins LB, Swenberg JA, Di Giulio RT (2009) Effects of benzo[a]pyrene on mitochondrial and nuclear DNA damage in Atlantic killifish (Fundulus heteroclitus) from a creosote-contaminated and reference site. Aquat Toxicol 95(1):44–51
Kim S, Johnson V, Sharma R (2003) Oral exposure to inorganic mercury alters T lymphocyte phenotypes and cytokine expression in BALB/c mice. Arch Toxicol 77(11):613–620
Livak K, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DeltaDeltaCT method. Methods 25:402–408
Murphy GW (2004) Uptake of mercury and relationship to food habits of selected fish species in the Shenandoah River basin. Virginia Virginia Polytechnic Institute and State University, Blacksburg
Nam D-H, Yates D, Ardapple P, Evers D, Schmerfeld J, Basu N (2012) Elevated mercury exposure and neurochemical alterations in little brown bats (Myotis lucifugus) from a site with historical mercury contamination. Ecotoxicology 21(4):1094–1101
Nascimento JLMD, Oliveira KRM, Crespo-Lopez ME, Macchi BM, Maués LAL, MDCN Pinheiro, Silveira LCL, Herculano AM (2008) Methylmercury neurotoxicity & antioxidant defenses. Indian J Med Res 128:373–382
Onyido I, Norris AR, Buncel E (2004) Biomolecule-mercury interactions: modalities of DNA base-mercury binding mechanisms. Remediation strategies. Chem Rev 104(12):5911–5929
Osborne CE, Evers DC, Duron M, Schoch N, Yates D, Buck D, Lane OP, Franklin J (2011) Mercury contamination within terrestrial ecosystems in New England and Mid-Atlantic States: [rofiles of soil, invertebrates, songbirds, and bats. Biodiversity Research Institute, Gorham
Pereira C, Guilherme S, Barroso C, Verschaeve L, Pacheco M, Mendo S (2010) Evaluation of DNA damage induced by environmental exposure to mercury in Liza aurata using the comet assay. Arch Environ Contam Toxicol 58(1):112–122
Santos JH, Meyer JN, Mandavilli BS, Houten BV (2006) Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. In: Henderson DS (ed) Methods in molecular biology: DNA repair protocols: mammalian systems, 2nd edn. Humana Press Inc, Totowa
Sharpe MA, Livingston AD, Baskin DS (2012) Thimerosal-derived ethylmercury is a mitochondrial toxin in human astrocytes: possible role of fenton chemistry in the oxidation and breakage of mtDNA. J Toxicol 2012:12
Sorenson MD (2003) Avian mtDNA primers (http://people.bu.edu/msoren/primers.html)
Sugg DW, Chesser RK, Brooks JA, Grasman BT (1995) The association of DNA damage to concentrations of mercury and radiocesium in largemouth bass. Environ Toxicol Chem 14(4):661–668
Taddei F, Scarcelli V, Frenzilli G, Nigro M (2001) Genotoxic hazard of pollutants in cetaceans: dNA damage and repair evaluated in the bottlenose dolphin (Tursiops truncatus) by the comet assay. Mar Pollut Bull 42(4):324–328
Varian-Ramos CW, Swaddle JP, Cristol DA (2014) Mercury reduces avian reproductive success and imposes selection: an experimental study with adult- or lifetime-exposure in zebra finch. PLoS ONE 9(4):e95674
Wada H, Cristol DA, McNabb FMA, Hopkins WA (2009) Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ Sci Technol 43(15):6031–6038
Wada H, Yates D, Evers D, Taylor R, Hopkins W (2010) Tissue mercury concentrations and adrenocortical responses of female big brown bats (Eptesicus fuscus) near a contaminated river. Ecotoxicology 19(7):1277–1284
Wang J, Newman MC, Xu X, Liang L (2013) Higher and more variable methylmercury biomagnification factors for floodplain than the contiguous river (South River, Virginia USA). Ecotoxicol Environ Saf 92:191–198
Weaver KN, Alfano SE, Kronquist AR, Reeder DM (2009) Healing rates of wing punch wounds in free-ranging little brown myotis (Myotis lucifugus). Acta Chiropt 11(1):220–223
Whitaker JO Jr (1995) Food of the big brown bat eptesicus fuscus from maternity colonies in Indiana and Illinois. Am Midl Nat 134(2):346–360
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17(2):146–160
Worthington-Wilmer J, Barratt E (1996) A non-lethal method of tissue sampling for genetic studies of chiropterans. Bat Res News 37(1):1–3
Yamane T, Davidson N (1961) On the complexing of desoxyribonucleic acid (DNA) by mercuric ion. J Am Chem Soc 83:2599–2607
Yates D, Angelo S, Divoll T, Evers DC (2012) Assessment of mercury exposure to bats at Onondaga Lake. Onondaga Lake, New York
Yates D, Adams E, Angelo S, Evers D, Schmerfeld J, Moore M, Kunz T, Divoll T, Edmonds S, Perkins C, Taylor R, O’Driscoll N (2014) Mercury in bats from the northeastern United States. Ecotoxicology 23(1):45–55
Zimmermann S (1999) The Development of a new approach to evaluate environmentally induced genetic damage in Hudson River biota. Bios 70(1):11–21
Zocche JJ, Leffa DD, Damiani AP, Carvalho F, Mendonça RÁ, dos Santos CEI, Boufleur LA, Dias JF, de Andrade VM (2010) Heavy metals and DNA damage in blood cells of insectivore bats in coal mining areas of Catarinense coal basin Brazil. Environ Res 110(7):684–691
Acknowledgments
Funding and support for this work was provided by the U.S. Fish and Wildlife Service, DuPont ™, the South River Science Team, and the U.S. Geological Survey. The use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the U.S. Fish and Wildlife Service. We would like to thank the many biologists that helped obtain samples for this study, Tim Divoll, Lucas Savoy, Dustin Meatty, Pedro Ardapple, Casey Huck, Patrick Keenan, Rick Reynolds (VADGF) and all the field technicians who put in countless hours. We also thank Catherine Maddox and Kelly Hallinger for their assistance in the laboratory.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karouna-Renier, N.K., White, C., Perkins, C.R. et al. Assessment of mitochondrial DNA damage in little brown bats (Myotis lucifugus) collected near a mercury-contaminated river. Ecotoxicology 23, 1419–1429 (2014). https://doi.org/10.1007/s10646-014-1284-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1284-9