Abstract
Tiger Grouper (Mycteroperca tigris) form fish spawning aggregations (FSAs) around the winter full moons (typically January through April) in the Caribbean. Males defend territories to attract mates in a lek-like reproductive strategy. Prior studies have documented rapid declines in populations with FSA-associated fisheries. This study examines the migratory behavior of adult male Tiger Grouper in Little Cayman, Cayman Islands, to better understand the impacts of aggregation fishing. As part of the Grouper Moon Project, we acoustically tagged ten spawning male Tiger Grouper at the western end of Little Cayman in February 2015. Using a hydrophone array surrounding the island, we tracked the movements of the tagged fish for 13 months. We observed 3 migratory strategies: resident fish (n = 2) that live at the FSA site, neighboring fish (n = 5) that live within 4 km of the site, and commuter fish (n = 3) that travel over 4 km for spawning. Fish began aggregating 2 days before the full moon and left 10–12 days after the full moon, from January to May. Regardless of migratory strategy, all tagged fish that aggregated after February 2015 returned to the west end FSA. However, in January 2016, one fish appeared to attend a different FSA closer to its presumed home territory. Tiger Grouper may establish multiple FSAs around Little Cayman, and males appear to attend FSAs near their home territories. Protracted spawning seasons, FSA site infidelity, and putative FSA catchments should all be considered to ensure sustainable fisheries management for this important species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tiger Grouper (Mycteroperca tigris) are a large reef fish common throughout the tropical West Atlantic, including the Caribbean, the Gulf of Mexico, and eastern Brazil (Heemstra and Randall 1993; Craig et al. 2011). Tiger Grouper reach sexual maturity around two years of age, and their natural life expectancy is at least nine years (Sadovy et al. 1994). Tiger Grouper are protogynous hermaphrodites, with males being larger than females, and they have a maximum total length (TL) of 100 cm (Heemstra and Randall 1993). Sex change can occur in fish ranging from 33 to 100 cm fork length (FL), although the length at which 50% of females change sex is approximately 52 cm FL (Caballero-Arango et al. 2013). They are largely piscivorous, and as such, they play an essential ecological role in structuring coral reef food webs (Randall 1987; Craig et al. 2011; Nemeth 2012). Depressed populations of Tiger Grouper may result in marked changes in reef fish community structure while reducing predation on invasive species, such as Indo-Pacific lionfish (Nemeth 2012). For the majority of the year, Tiger Grouper maintain territories on coral reefs and rocky bottom ecosystems between 10 and 40 m (Heemstra and Randall 1993). They are primarily ambush hunters but have also been seen foraging near the surface and are more likely to stray from cover than most other large-bodied groupers (Parrish 1987; Craig et al. 2011). Individuals stray from their home territories infrequently, although many make large-scale migrations in order to spawn (Farmer and Ault 2011; Domeier 2012).
Tiger Grouper form transient fish spawning aggregations (FSAs), where individuals leave their home territories and gather at predictable times and locations in order to reproduce (Domeier 2012). The locations of these FSAs are typically consistent, with small changes on the scale of 10s to 100s of meters from year to year (Colin 2012). In the central Caribbean, Tiger Grouper are known to form FSAs from January to April, in the days following the full moon (Sadovy et al. 1994). During these spawning windows, Tiger Grouper display a reproductive strategy similar to lekking behavior (Kirkpatrick and Ryan 1991). Large males aggressively defend 5–6 m territories from other males, while subdominant males maintain slightly smaller territories on the periphery of the spawning site (Sadovy et al. 1994, Starr et al. 2018). Sneaker males do not defend territory, but instead roam the FSA site looking for opportunities to join a spawning event. Females do not occupy territories at the site but spend their days underneath coral in and around the FSA before swimming up into the water column around sunset to form small spawning groups at the center of several male territories. Females indicate readiness to spawn by quivering, swimming upward rapidly, and releasing eggs, causing all males in a 3–5 m radius, including sneaker males, to chase after them and release sperm (Starr et al. 2018). Tiger Grouper display distinct sexual dichromatisms during spawning season, with males having uniformly dark pectoral fins and females having bright orange pectoral fins (Tuz-Sulub et al. 2006). Overall sex ratios at a Tiger Grouper FSA are around 1.4:1 male:female (White et al. 2002). Tiger Grouper FSAs are made up exclusively of sexually mature individuals with females ranging in size from 32 to 48 cm TL and males ranging from 36 to 64 cm TL (White et al. 2002).
While FSAs are reproductively advantageous for otherwise solitary and territorial individuals, they are also prime targets for fishing (Choat 2012; Molloy et al. 2012; Sadovy and Brule 2018). Tiger Grouper are of minor commercial interest, but their FSAs are heavily fished by artisanal fishers in some locations (Heemstra and Randall 1993; White et al. 2002; Matos-Caraballo et al. 2006). Typically, aggregating individuals are targeted by hook and line or spearfishing (Sadovy et al. 1994). FSA fishing can have significant negative impacts on spawning fish, including disrupting social and mating activities, altering population structure with respect to body size or sex ratios, and directly reducing the number of mature fish below sustainable levels (Sadovy de Mitcheson and Erisman 2012). FSAs are especially vulnerable to overfishing because catch-per-unit-effort can remain stable while overall population size declines, known as hyperstability (Domeier 2012). Once overexploited, most aggregations recover slowly or not at all (Domeier 2012; Stump et al. 2017). Globally, over half of all reported FSAs for all species are in decline, and nearly 10% are completely gone (Chollett et al. 2020). In the Caribbean and Gulf of Mexico, nearly all reported FSAs are in decline or extirpated (Nemeth 2012; Chollett et al. 2020). Tiger Grouper fisheries in Puerto Rico, Cuba, Mexico, and Honduras have experienced declines in the catch, and the species is thought to be declining regionally; however, more research is needed to determine the extent of the decline (Matos-Caraballo et al. 2006; Craig et al. 2011; Sadovy and Brule 2018).
Despite their clear ecological and economic importance, little is known about the spatial ecology of individual Tiger Grouper visiting FSAs. It is unclear (1) how much time an individual spends at an FSA site during the spawning season; (2) what the timing and variability of individual reproductive migrations is; (3) how large the catchment area of an FSA is (area including the home territories and migration routes of a population using a specific site (Nemeth 2012)); and (4) to what extent individuals are philopatric to specific FSAs across lunar cycles and years. Collectively, the answers to these questions support efforts to link the abundance of spawners at FSA sites to regional population dynamics for the purposes of population assessment (e.g., Waterhouse et al. 2020) and FSA fisheries risk assessment. To generate observations suitable for addressing the above questions, we took advantage of a whole island VEMCO VR2W hydrophone array surrounding Little Cayman, Cayman Islands, to track the movement of ten acoustically tagged adult male Tiger Grouper over a thirteen-month period. Our goal was to characterize the spatial ecology of individual Tiger Grouper in relation to spawning, providing valuable information on their behavior and thus support fisheries management efforts aimed at conserving and effectively managing the species.
Methods
Study site
This study focused on Tiger Grouper living in the waters surrounding Little Cayman, the smallest of the three islands that constitute the Cayman Islands (Fig. 1). A reef promontory on the west end of Little Cayman is home to a multi-species FSA where Tiger Grouper, as well as Nassau Grouper (Epinephelus striatus) and other reef fish (22 species of fish in 8 families; Whaylen et al. 2006), have been observed spawning (Whaylen et al. 2004; Whaylen et al. 2006). The reef has two terraces, one at 15 m and one at 33 m, with the deeper one featuring ridges of corals and sponges interspersed with valleys of sand about 30 m wide (Whaylen et al. 2004). Since 2002, scientists participating in the Grouper Moon Project (GMP), coordinated by Reef Environmental Education Foundation (REEF) and the Cayman Islands Department of Environment (CIDOE), have conducted research and monitoring activities in support of the CIDOE’s efforts to adaptively manage grouper populations (Waterhouse et al. 2020; Stock et al. 2021). Each winter, the GMP team collects natural history observations and various data products at the spawning site, coincident with the expected peak spawning period for Nassau Grouper. In 2015, the peak spawning period for Nassau Grouper occurred shortly after the February full moon. Peak spawning behavior for Tiger Grouper typically lags peak Nassau grouper by 2–3 days, such that the first night of Tiger Grouper spawning begins on the last of Nassau Grouper spawning. GMP research divers have observed Tiger Grouper spawning on up to 3 consecutive nights, although additional nights seem likely given that GMP research activities at the site typically wrap up before Tiger Grouper leave the spawning site.
Acoustic tagging
In February 2015, we tagged ten adult male Tiger Grouper with VEMCO V9-2 acoustic transmitters at the Little Cayman west end FSA (UCSD IACUC protocol number S12116). The transmitters were secured to Floy BFIM-69 Billfish tags using epoxy, and the divers tagged fish using pole spears over 3 days between February 4th and 6th (Fig. 2). Fish were tagged in situ rather than via hook-and-line or trapping because these latter two methods were ineffective for Tiger Grouper at the site during previous tagging efforts (i.e., no Tiger Grouper had ever previously been captured at the site with these methods). Because of the size of the V9-2 transmitters and the external attachment method, we opted to tag only the larger males in the spawning population, although lengths of these fish were not recorded due to the in situ method of tagging. This focus on large males somewhat limits our ability to describe the spatial ecology of Tiger Grouper spawning behavior as a whole, since previous studies have shown clear differences in spatial ecology between different size classes of grouper, with smaller fish typically being more active and making larger daily movements (Farmer and Ault 2011). The V9-2 transmitter has an estimated battery life of 419 days, or about thirteen months. In a coral reef environment, we assume the transmitters’ detection radius reached a maximum of 125 m, with a working detection range of 60–90 m (Welsh et al. 2012).
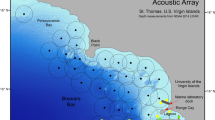
We monitored the movements of tagged fish using an array of eleven VR2W receivers surrounding Little Cayman (Fig. 3). These hydrophones recorded the time, date, and ID number of each transmitter detected. Tag detections in the array spanned 375 days (less than the putative battery life of the tags), starting on February 4th, 2015, and ending on February 14th, 2016. Of the eleven hydrophones, eight were deployed for all or most of the study period, while one was removed due to technical errors in September of 2015 and two were not deployed until January 2016 (Fig. 3). Of these partially deployed hydrophones, only one deployed in January 2016 detected a tagged Tiger Grouper. The study period included spawning seasons in 2015 and 2016 (partial), with spawning windows (consecutive lunar cycles) in February, March, April, and May of 2015, as well as January 2016.
Analysis
Tiger Grouper movement was quantified using daily detection frequency, or the number of times an individual tag was recorded by a receiver each day. For our initial analysis, detections were combined across all hydrophones to measure overall activity across time. Reproductive migration distances in fish that were detected away from the FSA were approximated based on the distance between the FSA and the halfway point between their last known location and the next farthest hydrophone. We then calculated the total distance traveled and average one-way migration distance (mean ± SD) per individual across all spawning windows. All analyses were done using R (version 4.1.1, R Core Team 2021).
Spawning windows were assumed to begin two days before the full moon and continue until 12 days after the full moon (Sadovy et al. 1994; White et al. 2002; Matos-Caraballo et al. 2006; Starr et al. 2018). For 2015, spawning windows were February 1–15, March 3–17, April 2–16, and May 1–13. In 2016, the study period only included one spawning window that lasted from January 21 to February 4. We corroborated these assumed spawning windows using our detections of tagged fish within the Little Cayman acoustic array.
Results
Of the ten tagged fish, only two, Fish 3 and 10, had detections for the entire study period. The majority of fish had their last detections after the March and April spawning windows of 2015, with the earliest final detection being Fish 6 in February 2015. Except for Fish 3 and 4, both of which remained at the west end Little Cayman FSA site for the entirety of the study period, none of the other fish were detected between the months of June 2015 and January 2016 anywhere in our acoustic receiver array (Fig. 4), likely due to the limited detection radius of the VEMCO V9-2 acoustic transmitters or tag loss.
Seven out of the ten tracked Tiger Grouper were detected only on the west end of the island. Fish 2, 5, 6, 7, and 9 were all detected at the FSA hydrophone only during spawning windows, suggesting that they lived near the FSA site but with home territories outside the detection range of the nearest hydrophone (4 km away). Fish 2, 5, 7, and 9 revisited the west end site during the spawning windows in February, March, and April of 2015. Fish 7 made an additional visit to the FSA site during the spawning window in May of 2015. Fish 6 was only detected during the February 2015 spawning window. Fish 3 and 4 were also only detected at the west end FSA site but were detected both in and out of the spawning season. These fish likely had home territories at the site and therefore did not migrate for spawning. Fish 3 had increased daily detections during the spawning seasons in February, March, and May of 2015, but its highest levels of daily detection were in the summer, with peaks in June and July. Fish 4 followed a similar pattern with increased detections during spawning season in February, March, and April and maximum daily detections in June of 2015.
Fish 1, 8, and 10 were the only tagged fish detected at hydrophones away from the FSA site. Following tagging, the average one-way migration distance across all spawning windows was 8 ± 3 km. After the spawning window in February 2015, both Fish 1 and Fish 8 remained near the west end FSA site, despite having apparent home territories elsewhere. Only Fish 10 left the area surrounding the site at the end of the February window. At the end of the March 2015 spawning window, Fish 1 migrated approximately 8.5 km to the south side of the island (Fig. 5). Fish 1 was not detected at any other hydrophone for the rest of the study period. Fish 8 made migrations during both the March and April spawning windows. At the end of March, Fish 8 migrated to the northwest side of Little Cayman, a distance of approximately 5.5 km. Fish 8 returned to the FSA site at the beginning of the April spawning window and migrated back to the same presumed home reef at the end of the spawning window for a total migration distance of over 16 km (Fig. 5).
Fish 10 covered the greatest distance during spawning, making the longest single migration at over 13 km and traveling the longest total distance of 61 km. At the end of the February spawning window, Fish 10 traveled about 9.2 km from the west end FSA site along the north side of the island. During the March spawning window, the fish returned to the FSA and subsequently made a 13.1 km trek back past Bloody Bay to the northeast side of the island at the conclusion of the spawning window. Fish 10 then returned to the FSA in April before migrating back to the northeast side of the island at the end of the spawning window. In both March and April, these one-way migrations were completed in a single day. In January of 2016, Fish 10 did not return to the west end FSA site, despite Tiger Grouper spawning activity being observed at the site by GMP divers. Instead, Fish 10 was detected near a hydrophone on the northeast end of Little Cayman for the duration of that spawning window (hydrophone 7; Fig. 3). Because hydrophone 7 was deployed in January 2016, it is not clear whether Fish 10 simply forewent spawning in this spawning window, or whether hydrophone 7 sits at a separate FSA visited by Fish 10 in January. Anecdotal reports of a Tiger Grouper FSA in this area from research divers on Little Cayman suggest that the latter may be the case (Alli Candelmo, pers. comm.). Detections at this location stopped six days after the full moon, which is consistent with previous studies of Tiger Grouper spawning behavior, showing that fish leave FSA sites as early as five days after the full moon (Starr et al. 2018).
All ten fish exhibited increased daily detections during spawning windows. In 2015, all individuals except for Fish 6 returned to the FSA site on the west end of Little Cayman for subsequent spawning windows. Fish began aggregating as early as three days before the full moon and typically left between ten and twelve days after, corroborating the spawning windows observed in previous studies (Sadovy et al. 1994; White et al. 2002; Matos-Caraballo et al. 2006; Starr et al. 2018). At the conclusion of a spawning window, most fish left the site within the same two-day period, although Fish 9 remained for an extra six days in February 2015 (Fig. 6). In May 2015, only Fish 7 returned to the FSA site and stayed for a four-day period starting four days after the full moon. In January 2016, Fish 10 was detected at a potential second FSA site on the east end of the island. The first detection occurred on the date of the full moon, and detections continued for six days after (Online resource 1).
Discussion
Understanding aggregating behaviors is a key part of assessing the connection between the abundance of aggregating fishes at FSAs and the regional populations of aggregating species. Among the ten Tiger Grouper we tagged, we observed three different behavior types in the visitors to the FSA site: residents, or those with home reefs at or near the site; neighbors, or those with home reefs farther from the site but no farther than the next closest hydrophone (~4 km); and commuters, or those with home reefs four or more kilometers away who were detected away from the site. Neighboring fish (Fish 2, 5, 6, 7, and 9) made up half of the tagged Tiger Grouper, with three commuter fish (Fish 1, 8, and 10) and two resident fish (Fish 3 and 4) making up the other half. The neighboring fish all had similar patterns of movement, visiting the FSA site only during spawning windows and returning to home territories between one and four times between February and May 2015. After the February 2015 spawning window, Fish 9 lingers at the FSA site longer than the other neighboring fish which may be evidence of a temporary staging area used by migrating fish to minimize travel within a spawning season (Nemeth 2012; Rowell et al. 2015). This is also supported by the fact that two commuter fish (Fish 1 and 8) were not detected leaving the FSA site after the February spawning window despite migrating in the following months. While we confirmed recurring aggregations each month from February to April, the return of Fish 7 to the aggregation site in May 2015 could be indicative of a longer spawning season than previously thought.
The one-way migration distances observed in this study, ranging from 5.5 to 13.1 km, are fairly short relative to those of other species surrounding larger islands. Nassau Grouper in the Bahamas, an island system with a much more extensive shelf area than Little Cayman, have been observed to travel between 70 and 260 km to reach an FSA site (Dahlgren et al. 2016; Stump et al. 2017), with some individuals making multiple spawning trips per season (Semmens et al. 2007; Dahlgren et al. 2016). Coral Trout in the northern Great Barrier Reef have been known to make one-way migrations of between 220 and 5210 km for spawning (Zeller 1998). Across sexes, Coral Trout made between 1 and 18 trips to an FSA site, although females were more likely to make only a single trip (Zeller 1998). In addition to being much smaller than the Bahamas or the Great Barrier Reef, Little Cayman is also isolated geomorphologically by the abyssal canyons surrounding the island (Jones 1994). As such, grouper have not been observed traveling between Little Cayman and the nearby islands for spawning despite their close proximity (Waterhouse et al. 2020). It is possible that Little Cayman’s small size and isolation allow Tiger Grouper to make short migrations, thus reducing the energetic demands of reproduction while allowing individuals relatively longer spawning seasons (Zeller 1998; Dahlgren et al. 2016; Stump et al. 2017). On the other hand, none of the fish we tagged took migration routes that spanned the length of the island (~16 km); most traveled less than 4 km.
The lack of detections outside of spawning season is likely because Tiger Grouper have relatively limited movement ranges while on their home reef during the majority of the year, usually only a few hundred square meters. It is important to note, however, that tag loss may have been a substantial factor in the lack of detections outside the spawning season (Carter et al. 1994; Afonso et al. 2016). Prior studies have estimated external tag retention rates of between 28 and 63%. It is thus possible that a number of our tags fell off before the batteries died (Sato et al. 2016; Runde et al. 2022). Indeed, some tags may be lost as early as two weeks, making it difficult to assess whether detections stop because of a lack of activity or because of tag loss (Runde et al. 2022). Because our hydrophones were several kilometers apart and had a limited detection radius (Welsh et al. 2012), the data we collected are insufficient to provide descriptions of Tiger Grouper movement patterns at any finer scale (e.g., territorial behavior).
Our two resident fish (Fish 3 and Fish 4) had somewhat synchronized movements over the study period, and both had maximum daily detection frequencies during the same period in June of 2015, after the spawning season. This is consistent with Keller et al. (2020) who showed that grouper make more movements per day in non-spawning months than during spawning months. It is possible that grouper do not feed or feed less during spawning, and a resumption of foraging behavior may account for the increase in movement during non-spawning months (Nemeth 2012). Groupers have been shown to have nearly identical daily routines with respect to feeding and movement (Carter et al. 1994; Koeck et al. 2014; Afonso et al. 2016). The lunar period can also influence detection rates, with brighter lunar periods leading to significantly higher activity (Farmer and Ault 2011). Seasonal variation in activity may be partially related to changes in water temperature, with previous studies on Dusky Grouper (Epinephelus marginatus) showing that fish move to shallower waters as temperatures increase in the summer (Koeck et al. 2014). Seasonal changes in environmental conditions have similar impacts on ambient noise from both biological and weather-related sources, which has been shown to influence the detectability of acoustic tags in coral reef environments (Lammers et al. 2008; Cagua et al. 2013). Increased detections in the summer could also be driven in part by shifting centers of activity within their home range after spawning, as is seen in Nassau Grouper (Blincow et al. 2020). The two resident fish likely have adjacent, or at least nearby, territories because they were both within range of the FSA hydrophone for the majority of the study, suggesting that these potential shifts in activity centers may be synchronized between fish in close proximity.
The commuter fish help us better define the catchment area of the west end FSA. While commuter fish traveled 5–13 km to attend the FSA, most tagged fish appeared to have territories closer to the spawning site. No commuter fish appeared to circumnavigate the island during the spawning window, and we found no evidence to suggest spawning individuals navigated indirect routes to the west end FSA (e.g., fish on the North side of the island passing by the eastern tip of Little Cayman en route to the west end FSA). None of the tagged fish apparently maintained home territories within 6 km of the eastern end of the island. It seems likely, then, that at least one other Tiger Grouper aggregation site exists on Little Cayman. This notion is supported by the prior observation of an FSA on the northeastern side of the island (Alli Candelmo, pers. comm.) and the fact that the behavior of Fish 10 in 2016 suggests the visitation of an alternate FSA in the same area. Nonetheless, further studies are needed in order to confirm this speculation, including efforts to locate additional FSAs, and, ideally, additional tagging at these locations.
Fish 10’s brief visit to a potential alternate spawning site in January 2016 suggests that some Tiger Grouper may not be philopatric to a specific FSA site across spawning seasons, which is in contrast to the typical behavior of other transient aggregators, such as Nassau Grouper that exhibit high fidelity to a specific FSA site (Colin 2012; Domeier 2012; Dahlgren et al. 2016). Why would Tiger Grouper have multiple FSAs on Little Cayman, while Nassau Grouper only have a single FSA? We believe the answer lies in the difference in spawning strategies between species. Nassau Grouper are mass spawners, and males become non-territorial at the spawning site. Male Tiger Grouper, on the other hand, maintain discrete and well-defended territories at the spawning site. It thus stands to reason that territory space for male Tiger Grouper territories at the spawning site is limiting, relative to the species’ carrying capacity on the island for the rest of the year. Additional FSAs would be the logical consequence of these differential carrying capacities. Note, however, that while male territories are presumably limited at the spawning site, the abundance of non-territorial females is likely not. This difference between sexes likely sets up disparities between aggregation sites in terms of access to females. That is, sites with fitter males will be attended by proportionally more females, which in turn will further attract fit males. On a seasonal basis, individual male Tiger Grouper may be balancing competitive interactions with other males versus access to spawning females in their determination of which FSA to attend. Of course, the above hypothesis is difficult to explore further without understanding the movements of female Tiger Grouper; such a study should be a future priority for this species. Finally, it is important to temper the conclusions drawn from the behaviors of this single-tagged individual. Given that the individual was clearly a behavioral outlier from the other tagged fish and given that we did not confirm the presence of an alternate FSA site in the 2016 spawning season, additional tagging studies and an expanded effort to survey the island for alternate Tiger Grouper FSAs are a necessary part of efforts to confirm FSA infidelity across years in the species.
FSAs are sometimes used as an opportunity to assess fish stocks because they are often the only time that all or most adults of solitary reef fishes are in one location and generally observable (Domeier 2012; Stock et al. 2021). The regional reproductive strategy of having several low-density spawning sites versus a single high-density site has implications for this type of assessment, management, and conservation. If multiple spawning sites exist in a region, and if individual fish potentially use multiple sites, some or most of which are undocumented, FSA-based assessments will only partially reflect the region’s population. Similarly, spatiotemporal FSA management will only protect a portion of the population. Moreover, evidence that individuals may alternate between FSA sites suggests that individuals receiving the benefit of such protections in one year may ultimately select unprotected sites in future years. Finally, if it is true that there are differences in male fitness and female densities across FSA sites, as hypothesized above, it stands to reason that the proportion of the Tiger Grouper regional population represented by any given FSA will change as a function of the size of the regional population and thus cannot be taken as a constant proxy for regional abundance.
Tiger Grouper have significant ecological and economic importance, but the lack of a clear understanding of the connection between FSA populations and regional populations has hindered the development of management strategies related to population assessment and harvest control at FSAs. Our observations suggest that Tiger Grouper FSAs may be more frequent across space than those of most other large reef-associated grouper species in the Caribbean. Nonetheless, at least some individual Tiger Grouper cover 10s of km to attend an FSA, suggesting that harvest at any given Tiger Grouper FSA represents a clear overfishing vulnerability in the face of unregulated aggregation-based fisheries.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Afonso P, Abecasis D, Santos RS, Fontes J (2016) Contrasting movements and residency of two serranids in a small Macaronesian MPA. Fish Res 177:59–70. https://doi.org/10.1016/j.fishres.2015.12.014
Blincow KM, Bush PG, Heppell SA et al (2020) Spatial ecology of Nassau grouper at home reef sites: using acoustic telemetry to track a large, long-lived epinephelid across multiple years (2005−2008). Mar Ecol Prog Ser 655:199–214. https://doi.org/10.3354/meps13516
Caballero-Arango D, Brulé T, Nóh-Quiñones V et al (2013) Reproductive biology of the Tiger Grouper in the southern Gulf of Mexico. Trans Am Fish Soc 142:282–299. https://doi.org/10.1080/00028487.2012.730111
Cagua EF, Berumen ML, Tyler EHM (2013) Topography and biological noise determine acoustic detectability on coral reefs. Coral Reefs 32:1123–1134. https://doi.org/10.1007/s00338-013-1069-2
Carter J, Marrow GJ, Pryor V (1994) Aspects of the ecology and reproduction of Nassau Grouper, Epinephelus striatus, off the coast of Belize, Central America. Proc Gulf Carib Fish Inst 43:65–111
Choat JH (2012) Spawning aggregations in reef fishes: ecological and evolutionary processes. In: Sadovy de Mitcheson Y, Colin P (eds) Reef fish spawning aggregations: biology, research and management. Springer, Dordrecht, pp 85–116
Chollett I, Priest M, Fulton S, Heyman WD (2020) Should we protect extirpated fish spawning aggregation sites? Biol Conserv 241:108395. https://doi.org/10.1016/j.biocon.2019.108395
Colin PL (2012) Timing and location of aggregation and spawning in reef fishes. In: Sadovy de Mitcheson Y, Colin P (eds) Reef fish spawning aggregations: biology, research and management. Springer, Dordrecht, pp 117–158
Craig MT, Sadovy De Mitcheson Y, Heemstra PC (2011) Groupers of the world: a field and market guide. NISC, Grahamstown, South Africa
Dahlgren CP, Buch K, Rechisky E, Hixon MA (2016) Multiyear tracking of Nassau Grouper spawning migrations. Mar Coast Fish 8:522–535. https://doi.org/10.1080/19425120.2016.1223233
Domeier ML (2012) Revisiting spawning aggregations: definitions and challenges. In: Sadovy de Mitcheson Y, Colin P (eds) Reef fish spawning aggregations: biology, research and management. Springer, Dordrecht, pp 1–20
Farmer NA, Ault JS (2011) Grouper and snapper movements and habitat use in Dry Tortugas, Florida. Mar Ecol Prog Ser 433:169–184. https://doi.org/10.3354/meps09198
Heemstra PC, Randall JE (1993) Groupers of the world (family Serranidae, subfamily Epinephelinae): an annotated and illustrated catalogue of the grouper, rockcod, hind, coral grouper and lyretail species known to date. Food Agric Organ United Nations 16:274–280
Jones B (1994) Geology of the Cayman Islands. The Cayman Islands, pp 13–49
Keller JA, Herbig JL, Morley D et al (2020) Grouper tales: use of acoustic telemetry to evaluate grouper movements at Western Dry Rocks in the Florida Keys. Mar Coast Fish 12:290–307. https://doi.org/10.1002/mcf2.10109
Kirkpatrick M, Ryan MJ (1991) The evolution of mating preferences and the paradox of the lek. Nature 350:33–38. https://doi.org/10.1038/350033a0
Koeck B, Pastor J, Saragoni G et al (2014) Diel and seasonal movement pattern of the Dusky Grouper Epinephelus marginatus inside a marine reserve. Mar Environ Res 94:38–47. https://doi.org/10.1016/j.marenvres.2013.12.002
Lammers MO, Brainard RE, Au WWL et al (2008) An ecological acoustic recorder (EAR) for long-term monitoring of biological and anthropogenic sounds on coral reefs and other marine habitats. J Acoust Soc Am 123:1720–1728. https://doi.org/10.1121/1.2836780
Matos-Caraballo D, Posada JM, Luckhurst BE (2006) Fishery-dependent evaluation of a spawning aggregation of Tiger Grouper (Mycteroperca tigris) at Vieques Island, Puerto Rico. Bull Mar Sci 79:1–16
Molloy PP, Côté IM, Reynolds JD (2012) Why spawn in aggregations? In: Sadovy de Mitcheson Y, Colin P (eds) Reef fish spawning aggregations: biology, research and management. Springer, Dordrecht, pp 57–83
Nemeth RS (2012) Ecosystem aspects of species that aggregate to spawn. In: Sadovy de Mitcheson Y, Colin P (eds) Reef fish spawning aggregations: biology, research and management. Springer, Dordrecht, pp 21–55
Parrish JD (1987) The trophic biology of snappers and groupers. In: Polovina JJ, Ralston S (eds) Tropical snappers and groupers: biology and fisheries management. Westview Press, Boulder, Colorado, pp 405–464
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/
Randall JE (1987) A preliminary synopsis of the groupers (Perciformes: Serranidae: Epinephelinae) of the Indo-Pacific region. In: Polovina JJ, Ralston S (eds) Tropical snappers and groupers: biology and fisheries management. Westview Press, Boulder, Colorado, pp 89–188
Rowell TJ, Nemeth RS, Schärer MT, Appeldoorn RS (2015) Fish sound production and acoustic telemetry reveal behaviors and spatial patterns associated with spawning aggregations of two Caribbean groupers. Mar Ecol Prog Ser 518:239–254. https://doi.org/10.3354/meps11060
Runde BJ, Buckel JA, Bacheler NM et al (2022) Evaluation of six methods for external attachment of electronic tags to fish: assessment of tag retention, growth and fish welfare. J Fish Biol 101:419–430. https://doi.org/10.1111/jfb.14989
Sadovy AY, Colin PL, Domeier ML (1994) Aggregation and spawning in the tiger grouper, Mycteroperca tigris (Pisces: Serranidae). Copeia 1994:511–516
Sadovy de Mitcheson Y, Erisman B (2012) Fishery and biological implications of fishing spawning aggregations, and the social and economic importance of aggregating fishes. In: Sadovy de Mitcheson Y, Colin P (eds) Reef fish spawning aggregations: biology, research and management. Springer, Dordrecht, pp 225–284
Sadovy Y, Brule T (2018) Mycteroperca tigris. The IUCN Red List of Threatened Species 2018: e.T44682A46914961. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T44682A46914961.en. Accessed on 27 April 2022
Sato T, Nanami A, Takebe T et al (2016) Retention rates of plastic-infusion-process passive integrated transponder tags and external tags in the White-streaked Grouper Epinephelus ongus. North Am J Fish Manag 36:116–121. https://doi.org/10.1080/02755947.2015.1114536
Semmens BX, Luke KE, Bush PG, et al (2007) Investigating the reproductive migration and spatial ecology of Nassau Grouper (Epinephelus striatus) on Little Cayman Island using acoustic tags – an overview. Proc 58th Gulf Caribb Fish Inst 191–198
Starr RM, Ballesteros E, Sala E, Llenas JM (2018) Spawning behavior of the Tiger Grouper (Mycteroperca tigris) in a Caribbean atoll. Environ Biol Fish 101:1641–1655. https://doi.org/10.1007/s10641-018-0813-4
Stock BC, Heppell SA, Waterhouse L et al (2021) Pulse recruitment and recovery of Cayman Islands Nassau Grouper (Epinephelus striatus) spawning aggregations revealed by in situ length-frequency data. ICES J Mar Sci 78:277–292. https://doi.org/10.1093/icesjms/fsaa221
Stump K, Dahlgren CP, Sherman KD, Knapp CR (2017) Nassau Grouper migration patterns during full moon suggest collapsed historic fish spawning aggregation and evidence of an undocumented aggregation. Bull Mar Sci 93:375–389. https://doi.org/10.5343/bms.2016.1042
Tuz-Sulub A, Brulé T, Cervera-Cervera K, Espinoza-Mendez JC (2006) Evidence for sexual dichromatisms in spawning aggregations of Yellowfin Grouper Mycteroperca venenosa and Tiger Grouper Mycteroperca tigris from the southern Gulf of Mexico. J Fish Biol 69:1744–1755. https://doi.org/10.1111/j.1095-8649.2006.01241.x
Waterhouse L, Heppell SA, Pattengill-Semmens CV et al (2020) Recovery of critically endangered Nassau Grouper (Epinephelus striatus) in the Cayman Islands following targeted conservation actions. Proc Natl Acad Sci USA 117:1587–1595. https://doi.org/10.1073/pnas.1917132117
Welsh JQ, Fox RJ, Webber DM, Bellwood DR (2012) Performance of remote acoustic receivers within a coral reef habitat: implications for array design. Coral Reefs 31:693–702. https://doi.org/10.1007/s00338-012-0892-1
Whaylen L, Pattengill-Semmens CV, Semmens BX et al (2004) Observations of a Nassau grouper, Epinephelus striatus, spawning aggregation site in Little Cayman, Cayman Islands, including multi-species spawning information. Environ Biol Fish 70:305–313. https://doi.org/10.1023/B:EBFI.0000033341.57920.a8
Whaylen L, Bush P, Johnson B et al (2006) Characterizing a spawning aggregation over a period of five consecutive years at Little Cayman, Cayman Islands. Gulf Caribb Fish Inst Proc:1–14
White DB, Wyanski DM, Eleby BM, Lilyestrom CG (2002) Tiger Grouper (Mycteroperca tigris): profile of a spawning aggregation. Bull Mar Sci 70:233–240
Zeller DC (1998) Spawning aggregations: patterns of movement of the Coral Trout Plectropomus leopardus (Serranidae) as determined by ultrasonic telemetry. Mar Ecol Prog Ser 162:253–263. https://doi.org/10.3354/meps162253
Acknowledgements
We thank the many research divers and volunteers who have participated in and supported the REEF/CIDOE Grouper Moon Project. We appreciate the field and documentation assistance of Dr. Guy Harvey and the Guy Harvey Ocean Foundation. We also thank the generous support and logistical assistance provided by local Cayman businesses and residents (including, but not limited to, Peter Hillenbrand, Little Cayman Beach Resort, Reef Divers, and Southern Cross Club). Funding was provided in part by the Disney Conservation Fund and REEF members. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-2038238. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures involving animals were approved under UCSD IACUC protocol number S12116.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(XLSX 16 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sleugh, T., McCoy, C.M., Pattengill-Semmens, C.V. et al. Migratory behavior of aggregating male Tiger Grouper (Mycteroperca tigris) in Little Cayman, Cayman Islands. Environ Biol Fish 106, 1195–1206 (2023). https://doi.org/10.1007/s10641-023-01399-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01399-w