Abstract
Climate models suggest that by 2100, maximum temperatures where many larval Pacific lamprey, Entosphenus tridentatus, rear now may approach 27–31 °C. Little information exists on whether larval Pacific lamprey can tolerate these temperatures. We used acclimated chronic exposure (ACE) and direct acute exposure (DAE) experiments to determine the water temperature that is lethal to larval Pacific lamprey and whether sublethal water temperatures influence larval burrowing behavior. After 30 days in ACE experiments, all larvae survived in temperatures averaging ≤ 27.7 °C, no larvae survived in temperatures averaging ≥ 30.7 °C and the ultimate upper incipient lethal temperature (UILT) was estimated to be 29.2 °C. After seven days in DAE experiments, all larvae survived in 27.1 °C, only larvae acclimated to 23.3 °C survived in 29.1 °C, and no larvae survived in temperatures ≥ 30.6 °C. Using a time to death analysis, estimates of the UILTs ranged from 27.5 to 30.2 °C and the ultimate UILT was estimated to be > 28.3 °C. Using a percent mortality analysis, estimates of the ultimate UILT were not definitive but indicated it may be > 30.2 °C. To burrow, larvae reared for 30 days at temperatures averaging 26.9–27.7 °C exhibited more total time, active time, and stops than those reared for 30 days at temperatures averaging 22.3–23.9 °C. Our findings suggest that larval Pacific lamprey may be resilient to the most likely temperature increase scenarios predicted by climate models. However, they may be vulnerable to the high-temperature increase scenarios and, relatively high but sublethal temperatures may impact the behavior, and ultimately survival, of larval Pacific lamprey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lampreys are an ancient group of jawless fishes that has existed for over 350 million years (Janvier 2007; Docker et al. 2015). Of the approximately 45 extant lamprey species, Pacific lamprey, Entosphenus tridentatus, has one of the widest distributional ranges, historically occurring along the Pacific Rim from Mexico to Japan (Kan 1975; Ruiz-Campos and Gonzalez-Guzman 1996; Renaud 2008). The basic biology and ecology of Pacific lamprey has been relatively well described (e.g., Pletcher 1963; Hammond 1979; Beamish 1980; Richards 1980; Beamish and Levings 1991), with recent investigations providing valuable information on the larval and juvenile life history stage (e.g., McGree et al. 2008; Jolley et al. 2012). In particular, Pacific lamprey is an anadromous species and individuals likely spend the majority of their life as larvae in freshwater streams (Whitesel et al. 2020). Although previously underestimated (Kan 1975), the cultural, ecological, economic, and scientific importance of Pacific lamprey has recently received significant attention (Close et al. 2002; Docker et al. 2015).
Throughout much of their historic range, Pacific lamprey are a species of conservation concern. It is clear that the migration of Pacific lamprey has been restricted by barriers (e.g., dams) and their distribution has contracted (Moser and Mesa 2009). The trend in the overall abundance of Pacific lamprey is difficult to assess. Although not panmictic, Pacific lamprey exhibit relatively little philopatry within their range (Spice et al. 2012). There is not a directed marine fishery for Pacific lamprey and information on ocean abundance is generally inferred from bycatch records (Clemens et al. 2019). As a result, the abundance of a Pacific lamprey population cannot be easily quantified by traditional methods of enumerating anadromous adults returning to their natal stream (e.g., Bradford and Irvine 2000) or from ocean harvest. However, it has been suggested that Pacific lamprey abundance has declined to a remnant of pre-1940s levels (Close et al. 2002) and, in 2003, a petition was submitted to the US government to list Pacific lamprey under the Endangered Species Act (Wicks-Arshack et al. 2018). The most obvious threat to the status of Pacific lamprey may be barriers to upstream passage (Jackson and Moser 2012; Goodman and Reid 2017; Jolley et al. 2018) which prevent Pacific lamprey from using a large portion of their historic habitat. Threats also include impaired passage from impingement and entrainment during the movement of larvae and juveniles downstream (Moser et al. 2015), habitat degradation, streamflow management, water quality (Wang and Schaller 2015), and predation (Arakawa and Lampman 2020). In addition, Pacific lamprey may be vulnerable to increasing temperatures associated with climate change (Wang et al. 2020). Changing climate conditions may be the most complex and uncertain threat to the status of Pacific lamprey (Wang et al. 2021). Currently, Pacific lampreys are being managed as an imperiled species (Moser and Close 2003) and receiving extraordinary efforts (Wang and Schaller 2015; Clemens and Wang 2021) to promote their conservation (Clemens et al. 2017; Lucas et al. 2021).
It has been well documented that the global climate is and has been changing at an unusual rate (Houghton et al. 1990). Changes to the climate include predictions of less stable but increasing air temperatures (Rosenzweig 1989). In an attempt to predict the impending change to temperature (Kirtman et al. 2013), numerous models have been developed, each model with some uncertainty and resulting in predictions that vary by region (Pierce et al. 2009). Given the current trend, it has been suggested that by 2100, global air temperatures may be 1–5 °C higher than 1900–1960 values (Meehl et al. 2007; Wuebbles et al. 2017). Associated with changes to air temperature, stream temperatures are also predicted to increase (Isaak et al. 2012; Arismendi et al. 2014). Currently, many of the freshwater locations where Pacific lamprey can be found naturally (e.g., Clemens et al. 2017) experience maximum water temperatures near 26 °C (USGS 2022a). With predictions that water temperatures may also rise 1–5 °C in the next 25–35 years (Wu et al. 2012), it is unclear whether maximum temperatures at many locations where they exist now will approach or exceed the lethal limit for Pacific lamprey.
Pacific lamprey, including the larval stage, are obligate ectotherms and are directly influenced by ambient water temperature. However, few studies have directly evaluated the thermal tolerance of Pacific lamprey. Embryo survival in Pacific lamprey appears greatest from 10 to 18 °C and is reduced at 22 °C (Meeuwig et al. 2005). Relative to thermal tolerance, lamprey embryos appear to be the least tolerant to warm water, whereas the larval through adult stages appear to be the most tolerant (Rodríguez-Muñoz et al. 2001). For non-embryonic stages, in a variety of species other than Pacific lamprey, evidence exists that optimal water temperatures may also be near 15–21 °C (see Farmer et al. 1977; Holmes and Lin 1994), while lethal water temperatures may occur near 27–31 °C (Potter and Beamish 1975; Macey and Potter 1978). Temperatures exceeding 27 °C may potentially be a lethal threshold for both adult and embryonic Pacific lamprey (Clemens et al. 2016).
How Pacific lamprey will respond to climate change is unclear. Recent reports have described the potential vulnerability of Pacific lamprey to climate change (Wang and Schaller 2015; Wang et al. 2020) but also raised the possibility that Pacific lamprey may be resilient to climate change (Wang et al. 2021). Since the majority of a Pacific lamprey’s life is likely spent as larvae in fresh water, this life history stage may be at the greatest risk from temperature increases associated with climate change. Ongoing conservation efforts would benefit from a characterization of larval Pacific lamprey sensitivity to increasing temperatures (Wang et al. 2020), which has not been well evaluated.
The goal of this investigation was to evaluate the ability of larval Pacific lamprey to tolerate water temperatures that may result from climate change. To begin to address this goal, this study had two objectives. The first objective was to determine the maximum temperature to which larval Pacific lamprey can acclimate, or ultimate upper incipient lethal temperature (ultimate UILT) (Fry et al. 1946; Brett 1952). The second objective was to determine whether a relatively high but sublethal temperature influences the burrowing behavior of larvae. We hypothesized that larvae reared in a temperature near their ultimate UILT would exhibit an impaired burrowing ability when compared to those reared in a significantly lower temperature, presumably near their preferred optimum.
Materials and methods
Animals
Larval Pacific lamprey were collected from the lowest reach (approximately 45.93573°N, − 122.61902°W, elevation 13 m) of Cedar Creek (Washington, USA) (Fig. 1). Larvae were captured by electrofishing (McGree et al. 2008), using only the 3 pulse/second current (Weisser and Klar 1990) to cause them to leave their burrows, after which they were netted and held in an aerated container of water from Cedar Creek. Captured larvae were anesthetized using buffered, tricaine methane sulfonate (MS-222, 50 mg/L) and measured (total length, mm). Both Pacific lamprey and western brook lamprey, Lampetra richardsoni, occupy Cedar Creek. Larvae longer than 60 mm were identified to genus using caudal characteristics (Docker et al. 2016). Only Entosphenus (Pacific lamprey) larvae (median 86 mm; range, 61–128 mm) were retained for the study. Experimental larvae were transported to a laboratory in aerated coolers. Transit was approximately 45 min.
Acclimated chronic exposure
Larvae (n = 100) were collected in June 2016, for use in an acclimated chronic exposure (ACE) experiment (Selong et al. 2001). Water temperature (henceforth temperature) in Cedar Creek during the collection event ranged from 16.6 to 19.1 °C. Upon arrival at the laboratory, individual larvae were randomly assigned and transferred to one of ten treatment tanks (10 larvae/tank). Treatment tanks were approximately 51 × 25 × 30 cm (length × width × depth) and a volume of approximately 37.9 L. Each tank contained approximately 5 cm (depth) of sand from Cedar Creek (particle size less than 0.5 mm in diameter) that was covered by approximately 15 cm (depth) of well water from the Vancouver Trout Hatchery. Initially, water in treatment tanks ranged from 15.3 to 17.2 °C with a dissolved oxygen range of 7.24–8.74 mg/L. All treatment tanks were held under a simulated natural photoperiod of artificial light (adjusted every 14 days). Each tank was covered and contained an active air stone, a submersible (50–75 W) heater (approximately 4 cm diameter × 24 cm length), a temperature logger (32 mm diameter × 16 mm height), and a pump that recirculated water at a rate of approximately 75 l/h through a mesh bag with activated charcoal.
The ACE experiment included five treatments, ultimately characterized by > 30 days exposure to approximately 21 °C, 24 °C, 27 °C, 30 °C, and 33 °C test temperatures. Each tank was randomly assigned to one of these five treatments, and each treatment was replicated (e.g., 21A and 21B). Each treatment tank experienced three phases of temperature profiles. During phase 1, larvae were acclimated to captive conditions. Temperature for all treatment tanks was held relatively constant (20.1–22.0 °C) for 12 days. During phase 2, larvae were acclimated to test temperatures. Temperatures in each treatment tank were adjusted over a period of eight days so that all tanks reached final test temperatures on the same date. The rate of temperature increase varied by test temperature, was relatively constant within a given tank, and did not exceed 1.5 °C/day for any tank. During phase 3, larvae were exposed to test temperatures which were held constant for over 40 days. To maintain exposure conditions, twice each week, well water was used to replace any water that evaporated from treatment tanks. Due to the high temperatures of some treatments and recirculating water, we were concerned that the addition of food to treatment tanks would result in fouling and anoxic conditions (Arakawa and Yanai 2021). Given this concern and reports that larval Pacific lamprey can survive for months with little to no external food, larvae were not fed for the duration of the experiment (Hokanson and Koenst 1986; McGree et al. 2008; Silver et al. 2009). Tanks were monitored daily, to remove mortalities when observed and ensure that dissolved oxygen remained > 4 mg/L (Randall 1970; Lampman et al. 2021; Schlosser et al. 2021). After 30 days of exposure to test temperatures, overall survival was determined. Live larvae were gently agitated from their burrows by running a probe through the sediment. When larvae emerged, they were captured and temporarily held in water from the respective test tank until no more live larvae were present in the test tank. Any larvae not captured at the end of the test period were presumed to have died during the test.
To determine whether temperature influenced survival, we assessed survival rates of larvae after 30 days in the various test temperatures. We used a Fisher’s exact test to compare two temperature treatments at a time, sequentially, from the lowest to highest survival rates. This resulted in nine planned comparisons, and a Bonferroni correction (Sokal and Rohlf 1995) was applied to the assessment. To estimate the ultimate UILT, we also used survival rates for each test temperatures (Zale 1984). Using the ACE method, ultimate UILT is characterized as the temperature survived by 50% of the population for the test period (Selong et al. 2001). In our experiments, larvae were generally burrowed and neither live nor dead larvae were readily observed during the experiment. Thus, to evaluate percent survival after 30 days as a function of test temperature, we used a symmetric sigmoidal regression
where S is percent survival, T is test temperature, and a, b, c, and d are regression coefficients. The parameters of the resulting equation were used to estimate the temperature that would result in 50% survival of the larvae after 30 days, or the ultimate UILT (30 days). We used an ANOVA to characterize and evaluate whether the total mass (measured to the nearest 0.01 g) of larvae in various groups were similar at the beginning of the experiment. For all statistical comparisons (here and throughout), differences were considered significant when P < 0.05 (for the ACE experiment, adjusted to P < 0.0056 when a Bonferroni correction was applied).
Direct acute exposure
Larvae were collected (n = 80–88 per collection) on September 15, 2016 (temperature 17.2 °C), and June 28 to July 17, 2017 (temperature 14.3–15.4 °C), for use in four direct acute exposure (DAE) experiments (Brett 1952; Kaya 1978; Lohr et al. 1996). These larvae did not exhibit characteristics indicating they were transforming to juveniles and none transformed during these experiments. In general, each experiment followed a similar protocol. Briefly, upon arrival at the laboratory, larvae experienced two phases of temperature profiles, an acclimation phase and a test phase. For the acclimation phase, individual larvae were randomly assigned and transferred to one of two acclimation tanks (40–44 larvae/tank). For a given experiment, we targeted acclimation temperatures approximating either 19 °C, 19 °C, 21 °C, or 25 °C. Tank set-up was as described for ACE experiments. Each acclimation tank contained approximately 3 cm (depth) of creek sand covered by approximately 15 cm (depth) of well water. The acclimation phase lasted for 7–9 days during which larvae were fed 0.05 g salmon analog/larvae (Jolley et al. 2015) on days 2 and 5. To maintain acclimation conditions, well water was used (days 2 and 5 of the acclimation phase) to replace any water that evaporated from acclimation tanks. After acclimation was complete, the test phase was initiated. A probe was used to gently agitate individual larvae from the sand of an acclimation tank after which they were captured, randomly assigned and directly transferred to one of four test tanks. Transfers occurred from approximately 08:30–09:30 h. Test tanks were randomly assigned to a temperature regime approximating either 27 °C, 29 °C, 31 °C, or 33 °C. For both 19 °C acclimation experiments and the 21 °C acclimation experiment, each test tank received 10–11 larvae. For the 25 °C acclimation experiment, one of the acclimation tanks failed and each test tank received five larvae. Test tanks were aerated but did not have sand or a pump and, otherwise, had similar conditions to those in acclimation tanks. During the test phase, larvae were exposed to test temperatures and held in test conditions for 168 h. Larvae were not fed during this phase of the experiment. Survival was determined at 14 time periods (1, 2, 3, 4, 6, 8, 12, 24. 48, 72, 96, 120, 144, and 168 h). To remove mortalities and ensure that dissolved oxygen remained > 4 mg/L, all tanks were monitored at each time interval. Specific variations among the four experiments (e.g., acclimation temperature) can be found in Table 1.
To estimate the upper incipient lethal temperature (UILT), or temperature at which 50% of the larvae from a given acclimation temperature survived the 7-day test period, we used two approaches. The most common approach analyzes the time to death (Brett 1952, Beitinger and Bennett 2000). Briefly, the analysis involved two steps. Initially, a symmetric sigmoidal regression (see Eq. 1) was used to evaluate the percent survival as a function of time in each test temperature. Based on the parameters of the regression equations, we estimated the time to which 50% of the larvae survived (LT50). For a given acclimation temperature, an exponential decay regression was then used to evaluate LT50 as a function of test temperature. The parameters of this regression equation were used to determine the UILT. As a complementary approach, we also analyzed percent mortality after 168 h. In our study, after 168 h in the five test temperatures, percent survival of each treatment was either 0% or 100%, with no intermediate survival values. Thus, to estimate the UILT using this approach, we averaged the warmest test temperature resulting in 100% survival with the coolest test temperature resulting in 0% survival. We used the UILT values to estimate the ultimate UILT (7 days) from each approach. Using the DAE method, ultimate UILT is commonly characterized by UILT values that no longer increase with increasing acclimation temperatures (Fry et al. 1946; Brett 1952; Beitinger and Bennett 2000) or the maximum temperature to which lamprey can be acclimated (Reynolds and Casterlin 1979). Thus, we plotted UILT values against acclimation temperature and attempted to estimate the ultimate UILT (7 days) as the temperature where UILT values reached a plateau (Fry 1971; Elliot 1981; Jobling 1981). We used an ANOVA to characterize and evaluate whether the total mass of larvae in various groups were similar.
Burrowing
Once survival was determined in ACE experiments, larvae from treatments 21A, 21B, 27A, and 27B were returned to their respective treatment tank. After 8 days of recovery from capture, these larvae were then used to evaluate burrowing behavior (Quintella et al. 2007; Dawson et al. 2015). Burrowing behavior was assessed over a period of 2 days (20 larvae/day). Larvae from each treatment were evaluated each day. Treatment tanks were randomly selected (e.g., larvae from 21A and 27B were evaluated on the first day of the trials). The order in which treatments were evaluated was also randomized (e.g., a larva from 21A was used in the first trial) and paired (e.g., a larva from 27B was evaluated in the second trial). Equal numbers of larvae from each treatment temperature were evaluated each day. Larvae were tested individually. Trials were conducted in two tanks (BT21 and BT27) that were the same temperature and dimensions as the corresponding treatment tanks. Tank set-up was as described for ACE experiments except that both tanks contained approximately 15 cm (depth) of sand from the Columbia River covered by approximately 10 cm (depth) of well water from the Vancouver Trout Hatchery. Trials were conducted from 07:00 to 13:00 h each day. During trials, temperature and dissolved oxygen in BT21 ranged from 21.3 to 22.3 °C and 6.05–7.06 mg/L, whereas temperature and dissolved oxygen in BT27 ranged from 26.7 to 28.3 °C and 5.20–5.40 mg/L.
To conduct a trial, a larva was gently agitated from the sand of a treatment tank, captured, and placed in a dark, covered, cylindrical container (approximately 75 mm in diameter) that contained approximately 150 mL of water from the corresponding burrowing tank. The larva was held in these conditions for 4 min then transferred to the respective burrowing tank through a 15- × 100-mm (diameter × length) plastic tube. To ensure that a larva would not encounter the tank wall once they started burrowing, the larva was guided through the tube into a bottomless, perforated, 16 l × 2 w × 20 h cm acrylic chamber located in the center of the burrowing tank. Once a larva touched the substrate, burrowing behavior was evaluated for up to 10 min or until the larva was completely burrowed. Each burrowing trial was recorded on video from which, total time, active time, inactive time, and the number of stops during burrowing were documented.
To determine whether temperatures influenced burrowing behavior, we compared the proportion of larvae from each treatment that burrowed within 10 min as well as total time, active time, inactive time, and number of stops (no. of times a larva was inactive for > 1 s) while burrowing (Dawson et al. 2015). Fisher’s exact test was used to compare the proportion of larvae that burrowed. Larvae that did not burrow (n = 1) were excluded from further analysis. The other four metrics were compared using a Mann–Whitney U-test. Initially, we compared replicate treatments (e.g., 21A to 21B). Since replicate treatments did not exhibit differences in any of the metrics, results from each replicate were pooled. Subsequent analysis compared all larvae from 21 °C to those from 27 °C treatment groups. We used Student’s t-test to characterize and evaluate whether the total mass of larvae in the two treatment groups were similar at the end of the burrowing trials.
Results
Acclimated chronic exposure
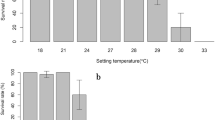
Once acclimated, actual mean temperatures during continuous exposure for 30 days in the 21 °C, 24 °C, 27 °C, 30 °C, and 33 °C treatment replicates were 22.3 °C and 23.9 °C, 23.9 °C and 24.1 °C, 26.9 °C and 27.7 °C, 30.7 °C and 31.1 °C, and 32.5 °C and 33.7 °C, respectively (Fig. 2). After 30 days of exposure, 100% of the larvae survived in the 21 °C, 24 °C, and 27 °C treatments, whereas 0% of the larvae survived in the 30 °C and 33 °C treatments. The survival rate (100%) of the 27 °C treatment was significantly higher than the survival rate (0%) of the 30 °C treatment (Fisher’s exact test, df = 1, P < 0.001). The ultimate UILT (30 d) was estimated to be 29.2 °C. The total mass of larvae placed in ACE treatments ranged from 0.44 to -3.20 g and was not significantly different between the 21 °C (mean = 1.04, 95% CI = 0.15), 24 °C (mean = 1.43, 95% CI = 0.21), 27 °C (mean = 1.24, 95% CI = 0.21), 30 °C (mean = 1.33, 95% CI = 0.27), or 33 °C (mean = 1.27, 95% CI = 0.30) treatment groups (ANOVA, df = 4, P = 0.212).
Temperatures to which larval Pacific lamprey were exposed during the Acclimated chronic exposure experiment. The acclimation phase occurred from − 20 to 0 day in the experiment. The treatment phase occurred from 0 to 30 day in the experiment. Final treatment temperatures were designed to be 21 °C, 24 °C, 27 °C, 30 °C, and 33 °C. Each treatment was duplicated (indicated by open and closed circles). For the treatment duplicates, actual mean treatment temperatures were 22.3 °C and 23.9 °C (line), 23.9 °C and 24.1 °C (dotted line), 26.9 °C and 27.7 °C (dashed line), 30.7 °C and 31.1 °C (dash-dash-dotted line), and 32.5 °C and 33.7 °C (dash-dot-dashed line), respectively
Direct acute exposure
The DAE experiments included four acclimation temperature experiments with actual mean temperatures ranging from 18.5 to 23.3 °C (Table 1). Each acclimation experiment included acute exposure to four test temperatures trials. Actual mean test temperatures ranged from 26.3 to 33.4 °C. After acclimation, all larvae survived for 168 h in test temperatures up to 27.1 °C. After acclimation to 23.3 °C, all larvae also survived for 168 h in a 29.1 °C test temperature. Survival exhibited a positive relationship to acclimation temperature, a negative relationship to test temperature (see Fig. 3 for example), and LT50 estimates ranged from 1.0–80.5 h. Using the time to death approach, the UILT estimates (see Fig. 4 for example) were positively related to acclimation temperature and ranged from 27.5 to 28.3 °C. After acclimation to 23.3 °C, only two LT50 values could be generated and the time to death approach could not be used to estimate the UILT. Using the percent mortality approach, the UILT estimates were also positively related to acclimation temperature and ranged from 27.7 to 30.2 °C. Overall, neither the time to death nor percent mortality approach yielded a plateau in UILT in relation to increasing acclimation temperature. Thus, we could not estimate a specific ultimate UILT (7 days). However, since UILT continued to increase with acclimation temperature, we were able to use both the time to death and percent mortality approach to estimate an ultimate UILT (7 days) of at least 28.3 °C and 30.2 °C, respectively. The total mass of larvae used in DAE trials ranged from 0.40 to 4.70 g. For a given acclimation temperature, larvae were randomly assigned to and statistically similar in size in each test group. For example, the mean total mass of larvae in the four test groups of the 19.8 °C acclimation trial were similar (ANOVA, df = 3, P = 0.665).
Survival curves from a direct acute exposure experiment. In this example, larvae were acclimated to 18.5 °C. A sigmoidal regression was used to estimate the LT50 as 43.1 h, 3.2 h, and 0.5 h in the 29.0 °C (dashed line, triangle), 31.1 °C (dash-dotted line, square), and 33.4 °C (dotted, diamond) test temperatures, respectively. All larvae survived in the 26.4 °C (solid line, circle) test temperature, and LT50 was not estimated. Survival was evaluated for 168 h. There were no changes in survival from 96 to 168 h (not shown)
Time to death estimate of the upper incipient lethal temperature (UILT) from a direct acute exposure experiment. In this example, larvae were acclimated to 18.5 °C. The data (diamond) were analyzed with a decaying exponential regression (line) and the resulting equation (UILT = (log e (LT50/1.01))/(− 2e14)) used to estimate the UILT as 27.5 °C. The 95% confidence interval around that estimate (dashed lines) ranged from 27.0 to 27.8 °C
Burrowing
After the completion of the 30-day ACE experiments, we evaluated the burrowing behavior of larvae from the 21 °C and 27 °C treatment groups. During the 30-day ACE experiments, actual mean temperatures in the 21 °C treatment replicates were 22.3 °C and 23.9 °C, while actual mean temperatures in the 27 °C treatment replicates were 26.9 °C and 27.7 °C. The proportion of larvae that burrowed was similar in the 21 °C (0.95) and 27 °C (1.00) treatment groups (Fisher’s exact test, P = 1.00). Total time to burrow (Fig. 5a) was significantly greater for larvae from the 27 °C treatment groups (67 s) than for those from the 21 °C treatment groups (16 s) (Mann–Whitney U-test, U = 108, P = 0.012). Active time during burrowing (Fig. 5b) was not different between larvae from the 27 °C treatment group (51 s) and the 21 °C treatment group (14 s) (Mann–Whitney U-test, U = 132, P = 0.066). Inactive time during burrowing (Fig. 5c) was significantly greater for larvae from the 27 °C treatment group (26 s) than for those from the 21 °C treatment group (2 s) (Mann–Whitney U-test, U = 80, P = 0.001). The number of stops during burrowing (Fig. 5d) was also significantly greater for larvae from the 27 °C treatment group (3) than for those from the 21 °C treatment group (1) (Mann–Whitney U-test, U = 69, P < 0.001). The total mass of larvae in the burrowing trials ranged from 0.47 to 2.48 g and was not significantly different between the 21 °C (mean = 1.21, 95% CI = 0.19) and 27 °C (mean = 1.16, 95% CI = 0.26) treatment groups (Student’s t-test, df = 18, P = 0.779).
Burrowing behavior of larval Pacific lamprey. Larvae were evaluated after rearing for 38 days in 21 °C (n = 19) or 27 °C (n = 20) test temperatures during an acclimated chronic exposure experiment. The behavior of each individual larva was evaluated from the time it contacted the sediment for up to 10 min (or until it was completely burrowed). Plots display median, interquartile range, and total range. Asterisk indicates a significant difference (Mann Whitney U test, P < 0.05)
Discussion
Larval Pacific lamprey were clearly able to tolerate temperatures warmer than 27 °C for extended periods and may be able to tolerate temperatures exceeding 30 °C for at least seven days. Several lines of evidence support this claim. After a gradual acclimation, all larvae were able to survive for 30 days, without exogenous food, in constant temperatures averaging as high as 27.7 °C. All larvae were also able to survive a constant temperature of 27.1 °C for seven days after being directly transferred from 19.8 °C. In addition, similar to studies on other species (e.g., Potter and Beamish 1975; Arakawa and Yanai 2021), the maximum temperature larvae that were able to tolerate were positively related to the temperature in which they were rearing. When acclimation temperature was 23.3 °C, all larvae in this study were able to survive for seven days after a direct transfer to a constant temperature of 29.1 °C. Furthermore, the maximum UILT (seven days) estimated from these experiments was 30.2 °C. In general, within the range that larval Pacific lampreys exist naturally (Orlov et al. 2008), these temperatures are similar to or exceed the instantaneous daily maximum temperatures that the warmest streams may approach (USGS 2022a). Given that most streams exhibit a circadian pattern of temperature, it is also informative to consider daily heat units (Allard et al. 2017). Since the test temperatures in this study were held constant, the maximum daily heat units, expressed as degree-days (UC 2014), that larvae were able to tolerate for at least seven consecutive days ranged from 27.7 to 29.1 °C. This number of daily heat units is also similar to or exceeds the maximum number of daily heat units larval Pacific lamprey would likely experience naturally. For example, it is not uncommon for most of the warmest streams where larvae rear to reach maximum temperatures that, currently, have a daily cycle ranging from approximately 25 to 30 °C. In this example, the number of daily heat units these larvae would experience approximates 27.5. This value is essentially equivalent to the daily heat units tolerated by larvae reared in this experiment at 27.7 °C and 5% lower than the daily heat units tolerated by larvae reared at 29.1 °C. Thus, the temperature larval Pacific lampreys that were able to tolerate appear to align well with what they experience naturally. To our knowledge, this is the first documentation of the maximum temperatures that larval Pacific lamprey can tolerate.
The temperature larval Pacific lampreys that were able to tolerate might be expected when considered from an evolutionary perspective. Although they may tend to remain in relatively large geographic regions (Hess et al. 2020), Pacific lamprey do not necessarily home to their natal stream (Goodman et al. 2008; Spice et al. 2012), and the geographical range of the interbreeding population is relatively expansive. The range in which Pacific lamprey exist is such that some larvae in the population likely experience maximum temperatures each year that reach or exceed 25 to 30 °C. While the heritability of heat tolerance in general is poorly understood (Beyer et al. 2011), there is some evidence that it may party heritable in fish (Beacham and Withler 1991). If the ability of larvae to tolerate heat is a heritable trait in Pacific lamprey, then it is reasonable to speculate that the warmest temperature larvae that can tolerate may reflect an adaptation to the warmest areas in the entirety of the population’s distribution.
The effects of temperature and oxygen availability are difficult to differentiate in many aquatic organisms. Water temperature is inversely related to dissolved oxygen levels and may often be limiting to fish as a function of reduced oxygen (Jonsson and Jonsson 2009). The life history of Pacific lamprey is such that larvae are often burrowed and sedentary, not swimming or actively holding position in the water column. If similar to other lamprey species (Manwell 1963; Bird et al. 1976), the larval blood of Pacific lamprey is likely to have a relatively high affinity for oxygen. The combination of their behavior and likely physiology would allow larval Pacific lamprey to require relatively low amounts of oxygen and be able to exist in hypoxic conditions for several hours (Lampman et al. 2021). This is a significant contrast to many potentially sympatric (Sloat and Reeves, 2014) or even inactive (Hill and Potter 1970; Morgan and Iwama 1998) teleosts. At water temperatures near 30 °C, it is not uncommon for streams where Pacific lamprey exist to have dissolved oxygen levels in the range of 5–6 mg/L (USGS 2022b), with streams < 30 °C often having dissolved oxygen levels > 8–10 mg/L (USGS 2022c). In part, larval Pacific lamprey may be able to tolerate relatively warm temperatures because oxygen does not become a limiting factor.
An ultimate UILT near 30 °C may be a common characteristic of many lamprey species. The ultimate UILT is typically defined as the maximum temperature to which a species can be acclimated (Fry et al. 1946; Brett 1952). The ultimate UILT estimated for larval Pacific lamprey in this study was at least 29.2 °C and possibly greater than 30.2 °C. This was evidenced both the ACE and DAE experiments, which complemented each other well. Since survival continued to increase with the increasing acclimation temperatures used in the DAE experiment, unfortunately, we were not able to definitively determine the maximum acclimation temperature above which survival would not improve. Nonetheless, the minimum values we estimated for larval Pacific lamprey are consistent with those reported for larvae of other lamprey species. For example, the estimated ultimate UILTs for larval sea lamprey, Petromyzon marinus, and European brook lamprey, Lampetra planeri (Potter and Beamish 1975), as well as European river lamprey, Lampetra fluviatilis (Golovanov et al. 2019; Smirnov et al. 2020), and Arctic lamprey, Lethenteron camtschaticum (Arakawa and Yanai 2021), range from 28 to 34.5 °C. Similarly, the ultimate UILT for pouched lamprey, Geotria australis, from the southern hemisphere has been estimated to be 28.3 °C (Macey and Potter 1978). Although the optimal or preferred temperature for larval lampreys may be significantly lower than their ultimate UILT (Holmes and Lin 1994), there are few to no reports of an ultimate UILT for larval lampreys that is less than 28 °C. Our description of the ultimate UILT for larval Pacific lamprey adds to the limited reports for other species and supports the notion that this characteristic may be relatively common and possibly conserved among all extant lampreys.
Although not lethal, relatively warm temperatures may have sublethal impacts on larval Pacific lamprey. In this experiment, larvae reared near 27 °C took longer to burrow, resting more often and for longer periods, than those reared near 21 °C. The specific relationship between temperature and the burrowing behavior of larval lampreys has not been well explored. However, other studies have shown that temperature can influence the behavior of larval lampreys. At temperatures exceeding 20 °C, Malmqvist and Brönmark (1982) found a negative effect on the filtration rate of small (< 2 g) larval European brook lamprey. In larval sea lamprey, Derosier et al. (2007) reported greater dispersal at relatively high temperatures as well as suppressed burrowing or reduced movement at relatively cold temperatures. Also, in sea lamprey, Reynolds and Casterlin (1979) found that larvae behaviorally thermoregulated by preferring temperatures between 13 and 14 °C. It has also been demonstrated that stressors can influence the burrowing behavior of larval lampreys. For example, similar to the burrowing response we observed, Dawson et al. (2015) reported that tagged sea lamprey larvae took more total time to burrow than untagged larvae. In addition, our finding is consistent with reports that temperature influences burrowing behavior in other aquatic species (Ouellette et al. 2004; Block et al. 2013). Burrowing is often considered one of the most energetically demanding forms of movement in aquatic organisms (Dorgan et al. 2011). The active metabolic rate in larval sea lamprey peaks near 21 °C and is reduced at 27 °C (Holmes and Lin 1994). If the physiology of larval Pacific lamprey is similar, those reared near 21 °C may have had the capacity to be more active and burrow more quickly than those reared near 27 °C. Interestingly, regardless of temperature, larval Pacific lamprey in this experiment had similar active times while burrowing. This is consistent with previous studies on larval lampreys which found that high temperatures do not influence all behaviors nor do stressors influence all aspects of burrowing. For example, temperatures exceeding 20 °C did not affect the filtration rate of relatively large brook lamprey larvae (Malmqvist and Brönmark 1982). In addition, the stress from being tagged did not cause the larvae studied by Dawson et al. (2015) to rest more during burrowing than untagged larvae. Thus, the sublethal effects of high temperature on larval burrowing behavior appear to be complex and the ultimate impact to the larvae is unclear.
While this study provides useful information toward understanding how larval Pacific lamprey respond to relatively warm temperatures, it is important to recognize its limitations. Although clinical trials have been used and can be important to understanding the basic biology of an organism (Piantadosi 2017), this study was conducted in a laboratory rather than the natural environment. In addition, following standard clinical procedures (Brett 1952; Selong et al. 2001), the temperatures we used were relatively constant and did not fluctuate daily as they would in a natural environment. In a natural environment, larval lampreys may have alternative approaches to coping with warm temperatures (Reynolds and Casterlin 1979). Furthermore, although the interbreeding population of Pacific lamprey (Spice et al. 2012) has an expansive range that includes many climates, there may be stream-specific differences in the larval response to temperature. Finally, since we evaluated larvae longer than 60 mm and collected from one stream, they may not be representative of all Pacific lamprey. A thorough understanding of how larval Pacific lamprey respond to warm temperatures requires further investigation.
Larval Pacific lamprey may be resilient to the temperature shifts predicted to occur as a result of climate change. Given the current models associated with climate change (Wu et al. 2012; Wuebbles et al. 2017), it appears that most streams in which larval Pacific lampreys exist are unlikely to experience temperatures exceeding 27 to 31 °C (USGS 2022a). In addition, these streams are unlikely to reach maximum temperatures for longer than 14 to 21 days. In this study, larvae were clearly able to tolerate temperatures exceeding 27 °C, for periods up to 30 days. Since Pacific lamprey may spend as much as 85% of their life as larvae in freshwater (Clemens et al. 2010; Hardisty 2013), this may be one of the most important life stages when assessing the overall resiliency of the species to climate change. Being able to tolerate a broad range of climactic conditions, including temperatures, is consistent with a 350-million-year evolutionary history (Docker et al. 2015). Alternatively, if the high increase scenarios predicted by some climate models occur (e.g., stream temperatures increasing 5 °C), larval Pacific lamprey may be vulnerable to the maximum temperatures that would occur (e.g. > 30 °C). In addition, for the species as a whole, it is unclear if warm temperatures at specific times during their development may be limiting to non-larval life stages (e.g., Meeuwig et al. 2005). Furthermore, larvae may be vulnerable to climate-driven changes other than temperature, such as hydrologic conditions (Wang et al. 2021). Finally, models may be underpredicting the increase in temperature that will result from climate change. Nonetheless, efforts to conserve Pacific lamprey would benefit from considering information specific to the species and not relying on information for other, sympatric species, which may have different thermal tolerance abilities (Wade et al. 2013).
Data availability
Data and material are stored by the US government and under the OPEN Government Data Act, upon request, can be made available to the public.
Code availability
Not applicable.
References
Allard DJ, Whitesel TA, Lohr SC, Koski ML (2017) Western pearlshell mussel life history in Merrill Creek, Oregon: reproductive timing, growth, and movement. Northwest Sci 91(1):1–14
Arakawa H, Lampman RT (2020) An experimental study to evaluate predation threats on two native larval lampreys in the Columbia River Basin, USA. Ecol Freshwater Fish 29(4):611–622
Arakawa H, Yanai S (2021) Upper thermal tolerance of larval Arctic lamprey (Lethenteron camtschaticum). Ichthyol Res 68(1):158–163
Arismendi I, Safeeq M, Dunham JB, Johnson SL (2014) Can air temperature be used to project influences of climate change on stream temperature? Environ Res Lett 9(8):084015
Beacham TD, Withler RE (1991) Genetic variation in mortality of Chinook salmon, Oncorhynchus tshawytscha (Walbaum) challenged with high water temperatures. Aquac Res 22:125–133
Beamish RJ (1980) Adult biology of the river lamprey (Lampetra ayresi) and the Pacific lamprey (Lampetra tridentata) from the Pacific coast of Canada. Can J Fish Aquat Sci 37:1906–1923
Beamish RJ, Levings CD (1991) Abundance and freshwater migrations of the anadromous parasitic lamprey, Lampetra tridentata, in a tributary of the Fraser River, British Columbia. Can J Fish Aquat Sci 48:1250–1263
Beitinger TL, Bennett WA (2000) Quantification of the role of acclimation temperature in temperature tolerance of fishes. Environ Biol Fish 58(3):277–288
Beyer J, Moy P, De Stasio B (2011) Acute upper thermal limits of three aquatic invasive invertebrates: hot water treatment to prevent upstream transport of invasive species. Environ Manag 47(1):67–76
Bird DJ, Lutz PL, Potter IC (1976) Oxygen dissociation curves of the blood of larval and adult lampreys (Lampetra fluviatilis). J Exp Biol 65(2):449–458
Block JE, Gerald GW, Levine TD (2013) Temperature effects on burrowing behaviors and performance in a freshwater mussel. J Freshw Ecol 28(3):375–384
Bradford MJ, Irvine JR (2000) Land use, fishing, climate change, and the decline of Thompson River, British Columbia, coho salmon. Can J Fish Aquat Sci 57(1):13–16
Brett JR (1952) Temperature tolerance in young Pacific salmon, genus Oncorhynchus. J Fish Board Can 9(6):265–323
Clemens BJ, Wang CJ (2021) Dispelling misperceptions of native lampreys (Entosphenus and Lampetra spp) in the Pacific Northwest (USA). Conserv Sci Pract 3(6):402
Clemens BJ, Binder TR, Docker MF, Moser ML, Sower SA (2010) Similarities, differences, and unknowns in biology and management of three parasitic lampreys of North America. Fisheries 35(12):580–594
Clemens BJ, Beamish RJ, Coates KC, Docker MF, Dunham JB, Gray AE, Wyss LA (2017) Conservation challenges and research needs for Pacific lamprey in the Columbia River basin. Fisheries 42(5):268–280
Clemens BJ, Weitkamp L, Siwicke K, Wade J, Harris J, Hess J, Orlov AM (2019) Marine biology of the Pacific lamprey Entosphenus tridentatus. Rev Fish Biol Fish 29(4):767–788
Clemens BJ, Schreck CB, Sower SA, Van de Wetering SJ (2016) The potential roles of river environments in selecting for stream- and ocean-maturing Pacific lamprey, Entosphenus tridentatus (Gairdner, 1836). In: Orlov AM, Beamish RJ (eds) Jawless fishes of the world, Cambridge Scholars Publishing, Chapman Hall, London, pp 299–322
Close DA, Fitzpatrick MS, Li HW (2002) The ecological and cultural importance of a species at risk of extinction. Pacific Lamprey Fisheries 27(7):19–25
Dawson HA, Potts DD, Maguffee AC, O’Connor LM (2015) Feasibility of passive integrated transponder technology to study in situ movements of larval sea lamprey. J Fish Wildl Manag 6(1):71–82
Derosier AL, Jones ML, Scribner KT (2007) Dispersal of sea lamprey larvae during early life: relevance for recruitment dynamics. Environ Biol Fish 78(3):271–284
Docker MF, Hume JB, Clemens BJ (2015) Introduction: a surfeit of lampreys. In: Docker MF (ed) Lampreys: biology, conservation and control. Springer, Dordrecht, pp 1–34
Docker MF, Silver GS, Jolley JC, Spice EK (2016) Simple genetic assay distinguishes lamprey genera Entosphenus and Lampetra: comparison with existing genetic and morphological identification methods. N Amer J Fish Manag 36(4):780–787
Dorgan KM, Lefebvre S, Stillman JH, Koehl MAR (2011) Energetics of burrowing by the cirratulid polychaete Cirriformia moorei. J Exp Biol 214(13):2202–2214
Elliot JM (1981) Some aspects of thermal stress in freshwater teleosts. In: Pickering AD (ed) Stress and fish. Academic Press, London, pp 209–245
Farmer GJ, Beamish FWH, Lett PF (1977) Influence of water temperature on the growth of the landlocked sea lamprey (Petromyzon marinus) and the associated host mortality. J Fish Res Bd Can 34:1373–1378
Fry FEJ, Hart JS, Walker KF (1946) Lethal temperature relations for a sample of young speckled trout. Biol Series Univ Toronto Studies Canada 54:9–35
Fry FEJ (1971) The effect of environmental factors on the physiology of fish In: Hoar WS, Randall DJ (eds) Fish physiology, Academic Press, London 6 1–98
Golovanov VK, Nekrutov NS, Zvezdin AO, Smirnov AK, Tsimbalov IA (2019) Thermoadaptation characteristics of European river lamprey Lampetra fluviatilis smolts. J Ichthyol 59:805–809
Goodman DH, Reid SB (2017) Climbing above the competition: innovative approaches and recommendations for improving Pacific Lamprey passage at fishways. Ecol Eng 107:224–232
Goodman DH, Reid SB, Docker MF, Haas GR, Kinziger AP (2008) Mitochondrial DNA evidence for high levels of gene flow among populations of a widely distributed anadromous lamprey Entosphenus tridentatus (Petromyzontidae). J Fish Biol 72(2):400–417
Hammond RJ (1979) Larval biology of the Pacific lamprey, Entosphenus tridentatus (Gairdner), of the Potlach River, Idaho. MS Thesis, University of Idaho
Hardisty MW (2013) Biology of the Cyclostomes. Springer, New York
Hess JE, Smith JJ, Timoshevskaya N, Baker C, Caudill CC, Graves D, Narum SR (2020) Genomic islands of divergence infer a phenotypic landscape in Pacific lamprey. Mol Ecol 29(20):3841–3856
Hill BJ, Potter IC (1970) Oxygen consumption in ammocoetes of the lamprey Ichthyomyzon hubbsi Raney. J Exp Biol 53:47–57
Hokanson KE, Koenst WM (1986) Revised estimates of growth requirements and lethal temperature limits of juvenile walleyes. Prog Fish-Cult 48(2):90–94
Holmes JA, Lin P (1994) Thermal niche of larval sea lamprey, Petromyzon marinus. Can J Fish Aquat Sci 51(2):253–262
Houghton JT, Jenkins GJ, Ephraums JJ (1990) Climate change: the IPCC scientific assessment. Amer Sci 80:6
Isaak DJ, Wollrab S, Horan D, Chandler G (2012) Climate change effects on stream and river temperatures across the northwest US from 1980–2009 and implications for salmonid fishes. Clim Change 113(2):499–524
Jackson A, Moser M (2012) Low-elevation dams are impediments to adult Pacific lamprey spawning migration in the Umatilla River. Oregon N Amer J Fish Manag 32(3):548–556
Janvier P (2007) Living primitive fishes and fishes from deep time. Fish Physiol 26:1–51
Jobling M (1981) Temperature tolerance and the final preferendum – rapid methods for the assessment of optimum growth temperatures. J Fish Biol 19:439–455
Jolley JC, Silver GS, Whitesel TA (2012) Occupancy and detection of larval Pacific lampreys and Lampetra spp in a large river: the Lower Willamette River. rans Amer Fish Soc 141:305–312
Jolley JC, Uh CT, Silver GS, Whitesel TA (2015) Feeding and growth of larval Pacific lamprey reared in captivity. N Amer J Aquac 77(4):449–459
Jolley JC, Silver GS, Harris JE, Whitesel TA (2018) Pacific lamprey recolonization of a Pacific Northwest river following dam removal. River Res Appl 34(1):44–51
Jonsson B, Jonsson N (2009) A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J Fish Biol 75(10):2381–2447
Kan TT (1975) Systematics, variation, distribution, and biology of lampreys of the genus Lampetra in Oregon. Dissertation, Oregon State University, Corvallis
Kaya CM (1978) Thermal resistance of rainbow trout from a permanently heated stream, and of two hatchery strains. Prog Fish-Cult 40:37–39
Kirtman B, Power SB, Adedoyin AJ, Boer GJ, Bojariu R, Camilloni I, Wang HJ (2013) Near-term climate change: projections and predictability. In: Climate Change 2013: The physical science basis, IPCC Working Group I, Contribution to AR5 953–1028
Lampman RT, Maine AN, Moser ML, Arakawa H, Neave FB (2021) Lamprey aquaculture successes and failures: a path to production for control and conservation. J Great Lakes Res 47:S201-215
Lohr SC, Byorth PA, Kaya CM, Dwyer WP (1996) High-temperature tolerances of fluvial Arctic grayling and comparisons with summer river temperatures of the Big Hole River, Montana. Trans Amer Fish Soc 125:933–939
Lucas MC, Hume JB, Almeida PR, Aronsuu K, Habit E, Silva S, Wang CJ, Zampatti B (2021) Emerging conservation initiatives for lampreys: research challenges and opportunities. J Great Lakes Res 47:S690-703
Macey DJ, Potter IC (1978) Lethal temperatures of ammocoetes of the southern hemisphere lamprey Geotria Australis Gray. Environ Biol Fish 3(2):241–243
Malmqvist B, Brönmark C (1982) Filter feeding in larval Lampetra planeri: effects of size, temperature and particle concentration. Oikos 38:40–46
Manwell C (1963) The blood proteins of cyclostomes: a study in phylogenetic and ontogenetic biochemistry. In: Brodal A, Fange R (eds) The biology of myxine. Universitetsforlaget, Oslo, pp 372–455
McGree M, Whitesel TA, Stone J (2008) Larval metamorphosis of individual Pacific lampreys reared in captivity. Trans Amer Fish Soc 137:1866–1878
Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Zhao ZC (2007) Global climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the Physical Science Basis, Contribution of: Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 747–846
Meeuwig MH, Bayer JM, Seelye JG (2005) Effects of temperature on survival and development of early life stage Pacific and western brook lampreys. Trans Amer Fish Soc 134:19–27
Morgan JD, Iwama GK (1998) Salinity effects on oxygen consumption, gill Na+, K+-ATPase and ion regulation in juvenile coho salmon. J Fish Biol 53(5):1110–1119
Moser ML, Close DA (2003) Assessing Pacific lamprey status in the Columbia River basin. Northwest Sci 77(2):116–125
Moser ML, Mesa MG (2009) Passage considerations for anadromous lampreys. In: Brown LR, Chase SD, Mesa MG, Beamish RJ, Moyle PB (eds) Biology, management, and conservation of lampreys in North America. American Fisheries Society, Bethesda, pp 115–124
Moser ML, Jackson AD, Lucas MC, Mueller RP (2015) Behavior and potential threats to survival of migrating lamprey ammocoetes and macrophthalmia. Rev Fish Biol Fish 25(1):103–116
Orlov AM, Savinyh VF, Pelenev DV (2008) Features of the spatial distribution and size structure of the Pacific lamprey Lampetra tridentata in the North Pacific. Russ J Mar Biol 34(5):276–287
Ouellette D, Desrosiers G, Gagne JP, Gilbert F, Poggiale JC, Blier PU, Stora G (2004) Effects of temperature on in vitro sediment reworking processes by a gallery biodiffusor, the polychaete Neanthes virens. Mar Ecol Prog Ser 266:185–193
Piantadosi S (2017) Clinical trials: a methodologic perspective. John Wiley & Sons
Pierce DW, Barnett TP, Santer BD, Gleckler PJ (2009) Selecting global climate models for regional climate change studies. Proc Nat Acad Sci U S A 106(21):8441–8446
Pletcher FT (1963) The life history and distribution of lampreys in the Salmon and certain other rivers in British Columbia. Thesis, University of British Columbia, Vancouver, Canada
Potter IC, Beamish FWH (1975) Lethal temperatures in ammocoetes of four species of lampreys. Acta Zool 56:85–91
Quintella BR, Andrade NO, Dias NM, Almeida PR (2007) Laboratory assessment of sea lamprey larvae burrowing performance. Ecol Freshw Fish 16(2):177–182
Randall DJ (1970) Gas exchange in fish. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 4. Academic Press, London, pp 253–292
Renaud CB (2008) Petromyzontidae, Entosphenus tridentatus: southern distribution record, Isla Clarión, Revillagigedo Archipelago. Mexico Check List 4(1):82–85
Reynolds WW, Casterlin ME (1979) Behavioral thermoregulation and the “final preferendum” paradigm. Amer Zool 19(1):211–224
Richards JE (1980) The freshwater life history of the anadromous Pacific lamprey, Lampetra tridentata. MSc Thesis, University of Guelph, Guelph
Rodríguez-Muñoz R, Nicieza AG, Brana F (2001) Effects of temperature on developmental performance, survival, and growth of sea lamprey embryos. J Fish Biol 58:475–486
Rosenzweig C (1989) Global climate change: predictions and observations. Amer J Agri Econ 71(5):1265–1271
Ruiz-Campos G, Gonzalez-Guzman S (1996) First freshwater record of Pacific lamprey, Lamptera tridentata, from Baja California, Mexico. Calif Fish Game 82:144–146
Schloesser N, Boogaard M, Johnson T, Kirkeeng C, Schueller J, Erickson R (2021) Use of an artificial stream to monitor avoidance behavior of larval sea lamprey in response to TFM and niclosamide. J Great Lakes Res 47:1192–1199
Selong JH, McMahon TE, Zale AV, Barrows FT (2001) Effect of temperature on growth and survival of bull trout, with application of an improved method for determining thermal tolerance in fishes. Trans Amer Fish Soc 130(6):1026–1037
Silver GS, Luzier CW, Whitesel TA (2009) Detection and longevity of uncured and cured visible implant elastomer tags in larval Pacific lampreys. N Amer J Fish Manag 29(5):1496–1501
Sloat MR, Reeves GH (2014) Individual condition, standard metabolic rate, and rearing temperature influence steelhead and rainbow trout (Oncorhynchus mykiss) life histories. Can J Fish Aquat Sci 71(4):491–501
Smirnov AK, Golovanov VK, Zvezdin AO, Golovanova IL, Kucheryavyy AV (2020) Unusual thermoregulatory behavior of anadromous and resident larvae of the river lamprey Lampetra fluviatilis (Petromyzontidae). Inland Water Biol 13(4):648–654
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. WH Freeman and Company, New York
Spice EK, Goodman DH, Reid SB, Docker MF (2012) Neither philopatric nor panmictic: microsatellite and mtDNA evidence suggests lack of natal homing but limits to dispersal in Pacific lamprey. Mol Ecol 21(12):2916–2930
UC (2014) University of California Agriculture and Natural Resources, statewide integrated pest management program, regents of the University of California. http://www.ipm.ucanr.edu. Accessed 14 July 2022
USGS (2022a) Real-time water quality, United States Geological Survey. https://waterwatch.usgs.gov/wqwatch/. Accessed 21 July 2022a
USGS (2022b) Temperature affects dissolved oxygen concentration, Unites States Geological Survey. https://www.usgs.gov/media/images/temperature-affects-dissolved-oxygen-concentrations. Accessed 21 July 2022b
USGS (2022c) Current water data, United States Geological Survey. https://waterdata.usgs.gov/nwis/current/?type=quality. Accessed 21 July 2022c
Wade AA, Beechie TJ, Fleishman E, Mantua NJ, Wu H, Kimball JS, Stanford JA (2013) Steelhead vulnerability to climate change in the Pacific Northwest. J Appl Ecol 50(5):1093–1104
Wang C, Schaller H (2015) Conserving Pacific lamprey through collaborative efforts. Fisheries 40(2):72–79
Wang CJ, Schaller HA, Coates KC, Hayes MC, Rose RK (2020) Climate change vulnerability assessment for Pacific lamprey in rivers of the western United States. J Freshw Ecol 35(1):29–55
Wang CJ, Hudson JM, Lassalle G, Whitesel TA (2021) Impacts of a changing climate on native lamprey species: from physiology to ecosystem services. J Great Lakes Res 47:S106-200
Weisser JW, Klar GT (1990) Electric fishing for sea lampreys (Petromyzon marinus) in the Great Lakes region of North America. In: Cowx I (ed) Developments in electric fishing. Cambridge University Press, Cambridge, pp 59–64
Whitesel TA, McGree M, Silver GS (2020) Predicting larval metamorphosis of Pacific lamprey Entosphenus tridentatus through measurements of length and mass. J Fish Biol 97(3):804–816
Wicks-Arshack A, Dunkle M, Matsaw S, Caudill C (2018) An ecological, cultural and legal review of Pacific lamprey in the Columbia River basin. Idaho L Rev 54:45
Wu H, Kimball JS, Elsner MM, Mantua N, Adler RF, Stanford J (2012) Projected climate change impacts on the hydrology and temperature of Pacific Northwest rivers. Water Resour Res 48:11
Wuebbles DJ, Fahey DW, Hibbard KA, DeAngelo B, Doherty S, Hayhoe K, Horton R, Kossin JP, Taylor PC, Waple AM, Weaver CP (2017) Executive summary. In: Wuebbles DJ, Fahey DW, Hibbard KA, Dokken DJ, Stewart BC, Maycock TK (eds) Climate science special report: Fourth national climate assessment, U.S. Global Change Research Program, Washington DC, 1 12–34
Zale AV (1984) Applied aspects of the thermal biology, ecology, and life history of the blue tilapia, Tilapia aurea (Pisces: Cichlidae). PhD Dissertation, University of Florida, Gainesville
Acknowledgements
Funding and support for this project were provided by the US Fish and Wildlife Service. We are grateful to the Columbia River Fish and Wildlife Conservation Office, particularly Larry Fishler, Jeff Jolley, Courtney Newlon, and Greg Silver for their invaluable assistance with numerous aspects of the project. We thank Melody Hollar for the help rearing and monitoring larvae during the experiments. We also appreciate the inspiration and perspective provided by James King and his colleagues at Inland Fisheries Ireland.
Funding
This research was funded by the US Fish and Wildlife Service.
Author information
Authors and Affiliations
Contributions
Timothy A. Whitesel and Christina T. Uh: design, implementation, analysis, writing.
Corresponding author
Ethics declarations
Disclaimer
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the US Fish and Wildlife Service.
Ethics approval
This research did not involve human subjects. All lamprey captures and handling were approved by the US Fish and Wildlife Service as well as the Washington Department of Fish and Wildlife under permit numbers JOLLEY 16–216 and WHITESEL 17–341.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Whitesel, T.A., Uh, C.T. Upper temperature limit of larval Pacific lamprey Entosphenus tridentatus: implications for conservation in a warming climate. Environ Biol Fish 106, 837–852 (2023). https://doi.org/10.1007/s10641-022-01372-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01372-z