Summary
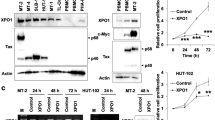
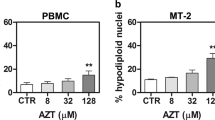
There is no cure for adult T cell leukemia/lymphoma (ATLL) associated with human T cell leukemia virus type 1 (HTLV-1), and novel targeted strategies are needed. NF-κB and AP-1 are crucial for ATLL, and both are transported to the nucleus by an importin (IPO)α/β heterodimeric complex to activate target genes. In this study, we aimed to elucidate the function of IPOβ1 in ATLL. The expression of IPOβ1 was analyzed by western blotting and RT-PCR. Cell growth, viability, cell cycle, apoptosis and intracellular signaling cascades were examined by the water-soluble tetrazolium-8 assay, flow cytometry and western blotting. Xenograft tumors in severe combined immune deficient mice were used to evaluate the growth of ATLL cells in vivo. IPOβ1 was upregulated in HTLV-1-infected T cell lines. Further, IPOβ1 knockdown or the IPOβ1 inhibitor importazole and the IPOα/β1 inhibitor ivermectin reduced HTLV-1-infected T cell proliferation. However, the effect of inhibitors on uninfected T cells was less pronounced. Further, in HTLV-1-infected T cell lines, inhibitors suppressed NF-κB and AP-1 nuclear transport and DNA binding, induced apoptosis and poly (ADP-ribose) polymerase cleavage, and activated caspase-3, caspase-8 and caspase-9. Inhibitors also mediated G1 cell cycle arrest. Moreover, the expression of NF-κB- and AP-1-target proteins involved in cell cycle and apoptosis was reduced. In vivo, the IPOα/β1 inhibitor ivermectin decreased ATLL tumor burden without side effects. IPOβ1 mediated NF-κB and AP-1 translocation into ATLL cell nuclei, thereby regulating cell growth and survival, which provides new insights for targeted ATLL therapies. Thus, ivermectin, an anti-strongyloidiasis medication, could be a potent anti-ATLL agent.









Similar content being viewed by others
References
Iwanaga M, Watanabe T, Yamaguchi K (2012) Adult T-cell leukemia: a review of epidemiological evidence. Front Microbiol 3:322
Ishitsuka K, Tamura K (2014) Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol 15:e517–e526
Hishizawa M, Kanda J, Utsunomiya A, Taniguchi S, Eto T, Moriuchi Y, Tanosaki R, Kawano F, Miyazaki Y, Masuda M, Nagafuji K, Hara M, Takanashi M, Kai S, Atsuta Y, Suzuki R, Kawase T, Matsuo K, Nagamura-Inoue T, Kato S, Sakamaki H, Morishima Y, Okamura J, Ichinohe T, Uchiyama T (2010) Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood 116:1369–1376
Qu Z, Xiao G (2011) Human T-cell lymphotropic virus: a model of NF-κB-associated tumorigenesis. Viruses 3:714–749
Gazon H, Barbeau B, Mesnard J-M, Peloponese J-M Jr (2018) Hijacking of the AP-1 signaling pathway during development of ATL. Front Microbiol 8:2686
Mori N, Fujii M, Ikeda S, Yamada Y, Tomonaga M, Ballard DW, Yamamoto N (1999) Constitutive activation of NF-κB in primary adult T-cell leukemia cells. Blood 93:2360–2368
Fujii M, Iwai K, Oie M, Fukushi M, Yamamoto N, Kannagi M, Mori N (2000) Activation of oncogenic transcription factor AP-1 in T cells infected with human T cell leukemia virus type 1. AIDS Res Hum Retrovir 16:1603–1606
Mori N (2009) Cell signaling modifiers for molecular targeted therapy in ATLL. Front Biosci 14:1479–1489
Hayden MS, Ghosh S (2004) Signaling to NF-κB. Genes Dev 18:2195–2224
Aggarwal A, Agrawal DK (2014) Importins and exportins regulating allergic immune responses. Mediat Inflamm 2014:476357
Stewart M (2007) Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol 8:195–208
Lee SJ, Sekimoto T, Yamashita E, Nagoshi E, Nakagawa A, Imamoto N, Yoshimura M, Sakai H, Chong KT, Tsukihara T, Yoneda Y (2003) The structure of importin-β bound to SREBP-2: nuclear import of a transcription factor. Science 302:1571–1575
Kutay U, Izaurralde E, Bischoff FR, Mattaj IW, Görlich D (1997) Dominant-negative mutants of importin-β block multiple pathways of import and export through the nuclear pore complex. EMBO J 16:1153–1163
Fagerlund R, Kinnunen L, Köhler M, Julkunen I, Melén K (2005) NF-κB is transported into the nucleus by importin α3 and importin α4. J Biol Chem 280:15942–15951
Liang P, Zhang H, Wang G, Li S, Cong S, Luo Y, Zhang B (2013) KPNB1, XPO7 and IPO8 mediate the translocation of NF-κB/p65 into the nucleus. Traffic 14:1132–1143
Forwood JK, Lam MHC, Jans DA (2001) Nuclear import of Creb and AP-1 transcription factors requires importin-β1 and ran but is independent of importin-α. Biochemistry 40:5208–5217
Harel A, Forbes DJ (2004) Importin beta: conducting a much larger cellular symphony. Mol Cell 16:319–330
Mosammaparast N, Pemberton LF (2004) Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol 14:547–556
Angus L, van der Watt PJ, Leaner VD (2014) Inhibition of the nuclear transporter, Kpnβ1, results in prolonged mitotic arrest and activation of the intrinsic apoptotic pathway in cervical cancer cells. Carcinogenesis 35:1121–1131
Martens-de Kemp SR, Nagel R, Stigter-van Walsum M, van der Meulen IH, van Beusechem VW, Braakhuis BJM, Brakenhoff RH (2013) Functional genetic screens identify genes essential for tumor cell survival in head and neck and lung cancer. Clin Cancer Res 19:1994–2003
van der Watt PJ, Maske CP, Hendricks DT, Parker MI, Denny L, Govender D, Birrer MJ, Leaner VD (2009) The Karyopherin proteins, Crm1 and Karyopherin β1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation. Int J Cancer 124:1829–1840
Zhu J, Wang Y, Huang H, Yang Q, Cai J, Wang Q, Gu X, Xu P, Zhang S, Li M, Ding H, Yang L (2016) Upregulation of KPNβ1 in gastric cancer cell promotes tumor cell proliferation and predicts poor prognosis. Tumor Biol 37:661–672
Smith ER, Cai KQ, Smedberg JL, Ribeiro MM, Rula ME, Slater C, Godwin AK, Xu X-X (2010) Nuclear entry of activated MAPK is restricted in primary ovarian and mammary epithelial cells. PLoS One 5:e9295
van der Watt PJ, Stowell CL, Leaner VD (2013) The nuclear import receptor Kpnβ1 and its potential as an anticancer therapeutic target. Crit Rev Eukaryot Gene Expr 23:1–10
Yang L, Hu B, Zhang Y, Qiang S, Cai J, Huang W, Gong C, Zhang T, Zhang S, Xu P, Wu X, Liu J (2015) Suppression of the nuclear transporter-KPNβ1 expression inhibits tumor proliferation in hepatocellular carcinoma. Med Oncol 32:128
Stelma T, Leaner VD (2017) KPNB1-mediated nuclear import is required for motility and inflammatory transcription factor activity in cervical cancer cells. Oncotarget 8:32833–32847
He S, Miao X, Wu Y, Zhu X, Miao X, Yin H, He Y, Li C, Liu Y, Lu X, Chen Y, Wang Y, Xu X (2016) Upregulation of nuclear transporter, Kpnβ1, contributes to accelerated cell proliferation- and cell adhesion-mediated drug resistance (CAM-DR) in diffuse large B-cell lymphoma. J Cancer Res Clin Oncol 142:561–572
Yan W, Li R, He J, Du J, Hou J (2015) Importin β1 mediates nuclear factor-κB signal transduction into the nuclei of myeloma cells and affects their proliferation and apoptosis. Cell Signal 27:851–859
Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y (1981) Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770–771
Yamamoto N, Okada M, Koyanagi Y, Kannagi M, Hinuma Y (1982) Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science 217:737–739
Popovic M, Sarin PS, Robert-Gurroff M, Kalyanaraman VS, Mann D, Minowada J, Gallo RC (1983) Isolation and transmission of human retrovirus (human T-cell leukemia virus). Science 219:856–859
Koeffler HP, Chen IS, Golde DW (1984) Characterization of a novel HTLV-infected cell line. Blood 64:482–490
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC (1980) Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A 77:7415–7419
Miyoshi I, Kubonishi I, Sumida M, Hiraki S, Tsubota T, Kimura I, Miyamoto K, Sato J (1980) A novel T-cell line derived from adult T-cell leukemia. Gan 71:155–156
Sugamura K, Fujii M, Kannagi M, Sakitani M, Takeuchi M, Hinuma Y (1984) Cell surface phenotypes and expression of viral antigens of various human cell lines carrying human T-cell leukemia virus. Int J Cancer 34:221–228
Maeda M, Shimizu A, Ikuta K, Okamoto H, Kashihara M, Uchiyama T, Honjo T, Yodoi J (1985) Origin of human T-lymphotrophic virus I-positive T cell lines in adult T cell leukemia: analysis of T cell receptor gene rearrangement. J Exp Med 162:2169–2174
Kaplan J, Tilton J, Peterson WD Jr (1976) Identification of T cell lymphoma tumor antigens on human T cell lines. Am J Hematol 1:219–223
Mori N, Prager D (1996) Transactivation of the interleukin-1α promoter by human T-cell leukemia virus type I and type II tax proteins. Blood 87:3410–3417
Tomayko MM, Reynolds CP (1989) Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 24:148–154
Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M (1986) Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J 5:2883–2888
Soderholm JF, Bird SL, Kalab P, Sampathkumar Y, Hasegawa K, Uehara-Bingen M, Weis K, Heald R (2011) Importazole, a small molecule inhibitor of the transport receptor importin-β. ACS Chem Biol 6:700–708
Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA (2012) Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J 443:851–856
Zhang C, Ao Z, Seth A, Schlossman SF (1996) A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J Immunol 157:3980–3987
Kamihira S, Atogami S, Sohda H, Momita S, Yamada Y, Tomonaga M (1994) Significance of soluble interleukin-2 receptor levels for evaluation of the progression of adult T-cell leukemia. Cancer 73:2753–2758
Nishioka C, Takemoto S, Kataoka S, Yamanaka S, Moriki T, Shoda M, Watanabe T, Taguchi H (2005) Serum level of soluble CD30 correlates with the aggressiveness of adult T-cell leukemia/lymphoma. Cancer Sci 96:810–815
Kuusisto HV, Wagstaff KM, Alvisi G, Roth DM, Jans DA (2012) Global enhancement of nuclear localization-dependent nuclear transport in transformed cells. FASEB J 26:1181–1193
Kosyna FK, Depping R (2018) Controlling the gatekeeper: therapeutic targeting of nuclear transport. Cells 7:221
Stelma T, Chi A, van der Watt PJ, Verrico A, Lavia P, Leaner VD (2016) Targeting nuclear transporters in cancer: diagnostic, prognostic and therapeutic potential. IUBMB Life 68:268–280
Iwanaga R, Ohtani K, Hayashi T, Nakamura M (2001) Molecular mechanism of cell cycle progression induced by the oncogene product tax of human T-cell leukemia virus type I. Oncogene 20:2055–2067
Iwanaga R, Ozono E, Fujisawa J, Ikeda MA, Okamura N, Huang Y, Ohtani K (2008) Activation of the cyclin D2 and cdk6 genes through NF-κB is critical for cell-cycle progression induced by HTLV-I tax. Oncogene 27:5635–5642
Kawakami H, Tomita M, Matsuda T, Ohta T, Tanaka Y, Fujii M, Hatano M, Tokuhisa T, Mori N (2005) Transcriptional activation of survivin through the NF-κB pathway by human T-cell leukemia virus type I tax. Int J Cancer 115:967–974
Wang C-Y, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr (1998) NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680–1683
Pahl HL (1999) Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18:6853–6866
Hess J, Angel P, Schorpp-Kistner M (2004) AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117:5965–5973
de Castro Barbosa ML, da Conceicao RA, Fraga AGM, Camarinha BD, de Carvalho Silva GC, Lima AGF, Cardoso EA, de Oliveira Freitas Lione V (2017) NF-κB signaling pathway inhibitors as anticancer drug candidates. Anti Cancer Agents Med Chem 17:483–490
Yamaguchi K, Matutes E, Catovsky D, Galton DAG, Nakada K, Takatsuki K (1987) Strongyloides stercoralis as candidate co-factor for HTLV-I-induced leukaemogenesis. Lancet 2:94–95
Zaha O, Hirata T, Uchima N, Kinjo F, Saito A (2004) Comparison of anthelmintic effects of two doses of ivermectin on intestinal strongyloidiasis in patients negative or positive for anti-HTLV-1 antibody. J Infect Chemother 10:348–351
Baraka OZ, Mahmoud BM, Marschke CK, Geary TG, Homeida MMA, Williams JF (1996) Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus. Eur J Clin Pharmacol 50:407–410
Acknowledgments
The authors would like to thank Fujisaki Cell Center, Hayashibara Biochemical Laboratories, Inc. for providing C5/MJ, HUT-102 and MT-1 cells, Dr. Naoki Yamamoto (Tokyo Medical and Dental University) for providing MT-2 and MT-4 cells, Dr. Diane Prager (UCLA School of Medicine) for providing SLB-1 cells, Dr. Michiyuki Maeda (Kyoto University) for providing ED-40515(−) cells, and Dr. Masahiro Fujii (Niigata University) for providing TL-OmI cells. Recombinant human IL-2 was kindly provided by Takeda Pharmaceutical Company Ltd. The measurement of protein concentrations was performed at the University of the Ryukyus Center for Research Advancement and Collaboration. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This study was supported partially by JSPS KAKENHI (17 K07175).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest is reported by the authors.
Ethical approval
All animal experiments were approved by the Animal Care and Use Committee of University of the Ryukyus (A2016149).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishikawa, C., Senba, M. & Mori, N. Importin β1 regulates cell growth and survival during adult T cell leukemia/lymphoma therapy. Invest New Drugs 39, 317–329 (2021). https://doi.org/10.1007/s10637-020-01007-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-01007-z