Abstract
Background
We investigated whether the photopic negative response (PhNR) in the electroretinogram (ERG) was affected in Parkinson’s disease (PD) patients and whether it was associated with retinal changes on optical coherence tomography (OCT).
Methods
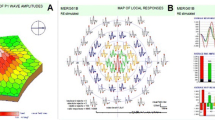
Thirty-two patients with PD and 31 age and sex-matched healthy controls from a single tertiary centre were included in the study. Hoehn and Yahr scale scores and the presence of REM sleep behaviour were recorded. PhNR, a-wave and b-wave responses in photopic ERG (red on blue background) and retinal layer thicknesses in OCT were obtained.
Results
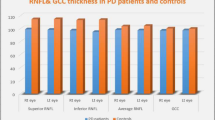
The mean age was 61 ± 10.4 in the PD group (female/male: 18/14) and 60.9 ± 7 in the control group (female/male: 18/13). The amplitudes of the PhNR, a- and b-waves in the ERG were significantly decreased in the PD group, but the implicit times were not significantly different. BCVA was significantly correlated with Hoehn and Yahr scores (p < 0.001, r = − 0.596). There was a significant correlation between BCVA and a-wave amplitude (p = 0.047, r = − 0.251). On OCT analysis, the thickness of the nasal INL was increased, and the temporal and inferior OPL and temporal peripapillary RNFL were decreased in the PD group compared to healthy controls (p = 0.032, p = 0.002, p = 0.016 and p = 0.012, respectively).
Conclusion
This study demonstrated reduced a-wave, b-wave and PhNR-wave amplitudes on ERG measurements in PD patients. These findings suggest that the whole ERG response, not just the PhNR, is attenuated in patient with PD, suggesting a possible involvement of the visual system in the disease.




Similar content being viewed by others
Data statement
The data that support the findings of this study are available from the corresponding author, Osman Ahmet Polat, upon reasonable request.
References
Chrysou A, Jansonius NM, van Laar T (2019) Retinal layers in Parkinson’s disease: a meta-analysis of spectral-domain optical coherence tomography studies. Parkinsonism Relat Disord 64:40–49. https://doi.org/10.1016/j.parkreldis.2019.04.023
Ahn J, Lee JY, Kim TW, Yoon EJ, Oh S, Kim YK et al (2018) Retinal thinning associates with Nigral dopaminergic loss in de novo Parkinson disease. Neurology 91(11):e1003–e1012. https://doi.org/10.1212/WNL.0000000000006157
Satue M, Rodrigo MJ, Obis J, Vilades E, Gracia H, Otin S et al (2017) Evaluation of progressive visual dysfunction and retinal degeneration in patients with Parkinson’s disease. Invest Ophthalmol Vis Sci 58(2):1151–1157. https://doi.org/10.1167/iovs.16-20460
Kaur M, Saxena R, Singh D, Behari M, Sharma P, Menon V (2015) Correlation between structural and functional retinal changes in Parkinson disease. J Neuroophthalmol 35(3):254–258. https://doi.org/10.1097/WNO.0000000000000240
Ortuno-Lizaran I, Sanchez-Saez X, Lax P, Serrano GE, Beach TG, Adler CH et al (2020) Dopaminergic retinal cell loss and visual dysfunction in Parkinson disease. Ann Neurol 88(5):893–906. https://doi.org/10.1002/ana.25897
Ortuno-Lizaran I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N (2018) Phosphorylated alpha-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord 33(8):1315–1324. https://doi.org/10.1002/mds.27392
Veys L, Vandenabeele M, Ortuno-Lizaran I, Baekelandt V, Cuenca N, Moons L et al (2019) Retinal alpha-synuclein deposits in Parkinson’s disease patients and animal models. Acta Neuropathol 137(3):379–395. https://doi.org/10.1007/s00401-018-01956-z
La Morgia C, Di Vito L, Carelli V, Carbonelli M (2017) Patterns of retinal ganglion cell damage in neurodegenerative disorders: parvocellular vs magnocellular degeneration in optical coherence tomography studies. Front Neurol 8:710. https://doi.org/10.3389/fneur.2017.00710
Huang L, Wang C, Wang W, Wang Y, Zhang R (2021) The specific pattern of retinal nerve fiber layer thinning in Parkinson’s disease: a systematic review and meta-analysis. J Neurol 268(11):4023–4032. https://doi.org/10.1007/s00415-020-10094-0
Ucak T, Alagoz A, Cakir B, Celik E, Bozkurt E, Alagoz G (2016) Analysis of the retinal nerve fiber and ganglion cell—inner plexiform layer by optical coherence tomography in Parkinson’s patients. Parkinsonism Relat Disord 31:59–64. https://doi.org/10.1016/j.parkreldis.2016.07.004
Tagliati M, Bodis-Wollner I, Yahr MD (1996) The pattern electroretinogram in Parkinson’s disease reveals lack of retinal spatial tuning. Electroencephalograph Clin Neurophysiol/Evoked Potentials Section 100(1):1–11. https://doi.org/10.1016/0168-5597(95)00169-7
Peppe A, Stanzione P, Pierelli F, De Angelis D, Pierantozzi M, Bernardi G (1995) Visual alterations in de novo Parkinson’s disease: pattern electroretinogram latencies are more delayed and more reversible by levodopa than are visual evoked potentials. Neurology 45(6):1144–1148. https://doi.org/10.1212/wnl.45.6.1144
Rangaswamy NV, Shirato S, Kaneko M, Digby BI, Robson JG, Frishman LJ (2007) Effects of spectral characteristics of ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci 48(10):4818–4828. https://doi.org/10.1167/iovs.07-0218
Frishman L, Sustar M, Kremers J, McAnany JJ, Sarossy M, Tzekov R et al (2018) ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol 136(3):207–211. https://doi.org/10.1007/s10633-018-9638-x
Asanad S, Felix CM, Fantini M, Harrington MG, Sadun AA, Karanjia R (2021) Retinal ganglion cell dysfunction in preclinical Alzheimer’s disease: an electrophysiologic biomarker signature. Sci Rep 11(1):6344. https://doi.org/10.1038/s41598-021-85010-1
Moss HE, Park JC, McAnany JJ (2015) The photopic negative response in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci 56(6):3709–3714. https://doi.org/10.1167/iovs.15-16586
Machida S (2012) Clinical applications of the photopic negative response to optic nerve and retinal diseases. J Ophthalmol. https://doi.org/10.1155/2012/397178
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with opticnerve atrophy. Arch Ophthalmol 122(3):341–346. https://doi.org/10.1001/archopht.122.3.341
Ortuno-Lizaran I, Esquiva G, Beach TG, Serrano GE, Adler CH, Lax P et al (2018) Degeneration of human photosensitive retinal ganglion cells may explain sleep and circadian rhythms disorders in Parkinson’s disease. Acta Neuropathol Commun 6(1):90. https://doi.org/10.1186/s40478-018-0596-z
Diaconu S, Falup-Pecurariu O, Tint D, Falup-Pecurariu C (2021) REM sleep behaviour disorder in Parkinson’s disease (review). Exp Ther Med 22(2):812. https://doi.org/10.3892/etm.2021.10244
Zhang X, Sun X, Wang J, Tang L, Xie A (2017) Prevalence of rapid eye movement sleep behavior disorder (RBD) in Parkinson’s disease: a meta and meta-regression analysis. Neurol Sci 38(1):163–170. https://doi.org/10.1007/s10072-016-2744-1
Sixel-Döring F, Trautmann E, Mollenhauer B, Trenkwalder C (2011) Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 77(11):1048–1054. https://doi.org/10.1212/WNL.0b013e31822e560e
Plazzi G (2004) REM sleep behavior disorders in Parkinson’s disease and other Parkinsonian disorders. Sleep Med 5(2):195–199. https://doi.org/10.1016/j.sleep.2004.01.003
Bhidayasiri R, Tarsy D. Parkinson’s disease: Hoehn and Yahr scale. Movement disorders: a video Atlas. Springer; 2012. p. 4–5. https://doi.org/10.1007/978-1-60327-426-5_2
Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH (2007) The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov Disord 22(16):2386–2393. https://doi.org/10.1002/mds.21740
Rufiange M, Dumont M, Lachapelle P (2005) Modulation of the human photopic ERG luminance-response function with the use of chromatic stimuli. Vision Res 45(17):2321–2330. https://doi.org/10.1016/j.visres.2005.02.010
Prencipe M, Perossini T, Brancoli G, Perossini M (2020) The photopic negative response (PhNR): measurement approaches and utility in glaucoma. Int Ophthalmol 40(12):3565–3576. https://doi.org/10.1007/s10792-020-01515-0
Armstrong RA (2011) Visual symptoms in Parkinson’s disease. Parkinsons Dis. https://doi.org/10.4061/2011/908306
Silverstein SM, Demmin DL, Schallek JB, Fradkin SI (2020) Measures of retinal structure and function as biomarkers in neurology and psychiatry. Biomark Neuropsych 2:100018. https://doi.org/10.1016/j.bionps.2020.100018
Nowacka B, Lubinski W, Honczarenko K, Potemkowski A, Safranow K (2015) Bioelectrical function and structural assessment of the retina in patients with early stages of Parkinson’s disease (PD). Doc Ophthalmol 131(2):95–104. https://doi.org/10.1007/s10633-015-9503-0
Nguyen-Legros J, Versaux-Botteri C, Vernier P (1999) Dopamine receptor localization in the mammalian retina. Mol Neurobiol 19(3):181–204. https://doi.org/10.1007/BF02821713
Nowacka B, Lubiński W, Honczarenko K, Potemkowski A, Safranow K (2015) Bioelectrical function and structural assessment of the retina in patients with early stages of Parkinson’s disease (PD). Doc Ophthalmol 131(2):95–104. https://doi.org/10.1007/s10633-015-9503-0
Ikeda H, Head G, Ellis CK (1994) Electrophysiological signs of retinal dopamine deficiency in recently diagnosed Parkinson’s disease and a follow up study. Vision Res 34(19):2629–2638. https://doi.org/10.1016/0042-6989(94)90248-8
Mello LGM, Paraguay IBB, Andrade TS, Rocha A, Barbosa ER, Oyamada MK et al (2022) Electroretinography reveals retinal dysfunction in Parkinson’s disease despite normal high-resolution optical coherence tomography findings. Parkinsonism Relat Disord 101:90–95. https://doi.org/10.1016/j.parkreldis.2022.06.018
Polo V, Satue M, Rodrigo MJ, Otin S, Alarcia R, Bambo MP et al (2016) Visual dysfunction and its correlation with retinal changes in patients with Parkinson’s disease: an observational cross-sectional study. BMJ Open 6(5):58. https://doi.org/10.1136/bmjopen-2015-009658
Huang L, Zhang D, Ji J, Wang Y, Zhang R (2021) Central retina changes in Parkinson’s disease: a systematic review and meta-analysis. J Neurol 268(12):4646–4654. https://doi.org/10.1007/s00415-020-10304-9
Zhou WC, Tao JX, Li J (2021) Optical coherence tomography measurements as potential imaging biomarkers for Parkinson’s disease: A systematic review and meta-analysis. Eur J Neurol 28(3):763–774. https://doi.org/10.1111/ene.14613
Garcia-Martin E, Larrosa JM, Polo V, Satue M, Marques ML, Alarcia R et al (2014) Distribution of retinal layer atrophy in patients with Parkinson disease and association with disease severity and duration. Am J Ophthalmol 157(2):470–8 e2. https://doi.org/10.1016/j.ajo.2013.09.028
Albrecht P, Muller AK, Sudmeyer M, Ferrea S, Ringelstein M, Cohn E et al (2012) Optical coherence tomography in parkinsonian syndromes. PLoS ONE 7(4):e34891. https://doi.org/10.1371/journal.pone.0034891
Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S (2014) alpha-synuclein in the inner retina in parkinson disease. Ann Neurol 75(6):964–966. https://doi.org/10.1002/ana.24182
Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB et al (2014) Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 71(5):589–595. https://doi.org/10.1001/jamaneurol.2014.65
Joyce DS, Feigl B, Kerr G, Roeder L, Zele AJ (2018) Melanopsin-mediated pupil function is impaired in Parkinson’s disease. Sci Rep 8(1):7796. https://doi.org/10.1038/s41598-018-26078-0
Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD (2000) A novel human opsin in the inner retina. J Neurosci 20(2):600–605. https://doi.org/10.1523/JNEUROSCI.20-02-00600.2000
Ostrin LA, Strang CE, Chang K, Jnawali A, Hung LF, Arumugam B et al (2018) Immunotoxin-induced ablation of the intrinsically photosensitive retinal ganglion cells in rhesus monkeys. Front Neurol 9:1000. https://doi.org/10.3389/fneur.2018.01000
Mandrekar JN (2010) Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 5(9):1315–1316. https://doi.org/10.1097/JTO.0b013e3181ec173d
Popova E (2014) Role of dopamine in distal retina. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 200(5):333–358. https://doi.org/10.1007/s00359-014-0906-2
Popova E, Kostov M, Kupenova P (2016) Effects of dopamine D(1) receptor blockade on the ERG b- and d-waves during blockade of ionotropic GABA receptors. Eye Vis 3:32. https://doi.org/10.1186/s40662-016-0064-4
Ikeda H, Head GM, Ellis CJ (1994) Electrophysiological signs of retinal dopamine deficiency in recently diagnosed Parkinson’s disease and a follow up study. Vis Res 34(19):2629–2638. https://doi.org/10.1016/0042-6989(94)90248-8
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no relevant financial interest to disclose.
Author information
Authors and Affiliations
Contributions
Conceptualization was contributed by [OAP, HŞ]; data curation was contributed by [MG, FO]; formal analysis was contributed by [HS]; investigation was contributed by [HS]; methodology was contributed by [HS, HA]; project administration was contributed by [HA]; software was contributed by [HS]; supervision was contributed by [HA]; validation was contributed by [OAP]; visualisation was contributed by [HA]; writing—original draft, was contributed by [OAP, HS]; writing—review and editing was contributed by [MG, FO, HA].
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethic approval
All procedures performed in human participants were in accordance with the ethical standards of the Erciyes University Local Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Study protocol was approved by Erciyes University Local Ethics Committee (No: 2021/566).
Informed consent
Written informed consent was obtained from all individual participants included in the study. Statement on the welfare of animals: No animals were used in this research.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statement on the welfare of animals
No animals were used in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Polat, O.A., Gultekin, M., Sener, H. et al. Retinal dysfunction in Parkinson’s disease—results of the extended protocol for photopic negative response (PHNR) full-field electroretinogram (ERG). Doc Ophthalmol 147, 89–98 (2023). https://doi.org/10.1007/s10633-023-09945-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-023-09945-8