Abstract
Purpose
Frequency-domain measures were applied to characterize neural deficits in individuals with schizophrenia using transient visual evoked potentials (tVEP). These measures were compared with conventional time-domain measures to elucidate underlying neurophysiological mechanisms and examine the value of frequency analysis.
Methods
Four frequency bands of activity identified in previous work were explored with respect to magnitude (spectral power), timing (phase), a combined measure, magnitude-squared coherence (MSC), and compared to amplitudes and times of prominent deflections in the response.
Results
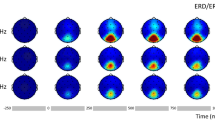
Band 2 power/MSC (14–28 Hz) captured the major deflections in the waveform and its power predicted N75-P100 amplitude for patients and controls. Band 3 power/MSC (30–40 Hz) correlated highly with the earliest deflection (P60-N75), reflecting input to primary visual cortex (V1) and produced the largest magnitude effect. Phase of the 24th harmonic component predicted P100 peak time for patients and controls and yielded the largest group difference. Cluster analyses including time- and frequency-domain measures identified subgroups of patients with differential neurophysiological effects. A small but significant difference in visual acuity was found between groups that appears to be neurally based: Acuity (range 0.63–1.6) was not correlated with any tVEP measures in controls nor with input timing to V1 (P60 peak time) in patients, but was correlated with later tVEP measures in patients. All but two of the patients were on antipsychotic medication: Medication level (chlorpromazine equivalents) was correlated negatively with tVEP time measures and positively with certain magnitude measures yielding responses similar to controls at high levels.
Conclusions
Overall, frequency-domain measures were shown to be objective and recommended as an alternative to conventional, subjective time-domain measures for analyzing tVEPs and in distinguishing between groups (patients vs. controls and patient subgroups). The findings implicated a loss of excitatory input to V1 in schizophrenia. Acuity as measured in the current study reflected disease status, and medication level was associated with improved tVEP responses. These novel tVEP techniques may be useful in revealing neurophysiological processes affected in schizophrenia and as a clinical tool.



Similar content being viewed by others
Data availability
Data used in this study will be made available upon request.
Notes
Neucodia system is currently marketed as EvokeDx, Konan Medical, USA.
References
McGhie A, Chapman J (1961) Disorders of attention and perception in early schizophrenia. Br J Med Psychol 34(2):103–116
Silverstein SM (2016) Visual perception disturbances in schizophrenia: a unified model. In: Li M, Spaulding WD (eds) The neuropsychopathology of schizophrenia: molecules, brain systems, motivation, and cognition. Springer, New York, pp 77–132
Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO et al (2005) Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry 62(5):495–504. https://doi.org/10.1001/archpsyc.62.5.495
Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G et al (2007) Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain 130(Pt 2):417–430. https://doi.org/10.1093/brain/awl233
Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J et al (2001) Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry 158(7):1126–1133
Hever F, Sahin D, Aschenbrenner S, Bossert M, Herwig K, Wirtz G et al (2021) Visual N80 latency as a marker of neuropsychological performance in schizophrenia: evidence for bottom-up cognitive models. Clin Neurophysiol 132(4):872–885. https://doi.org/10.1016/j.clinph.2021.01.007
Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC (2005) Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophr Res 76(1):55–65. https://doi.org/10.1016/j.schres.2004.10.011
Nunez D, Rauch J, Herwig K, Rupp A, Andermann M, Weisbrod M et al (2013) Evidence for a magnocellular disadvantage in early-onset schizophrenic patients: a source analysis of the N80 visual-evoked component. Schizophr Res 144(1–3):16–23. https://doi.org/10.1016/j.schres.2012.12.007
Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M et al (2005) Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol 116(9):2204–2215. https://doi.org/10.1016/j.clinph.2005.06.013
Silverstein SM, Rosen R (2015) Schizophrenia and the eye. Schizophr Res Cogn 2(2):46–55. https://doi.org/10.1016/j.scog.2015.03.004
Zemon V, Herrera S, Gordon J, Revheim N, Silipo G, Butler PD (2021) Contrast sensitivity deficits in schizophrenia: a psychophysical investigation. Eur J Neurosci 53(4):1155–1170. https://doi.org/10.1111/ejn.15026
Romani A, Zerbi F, Mariotti G, Callieco R, Cosi V (1986) Computed tomography and pattern reversal visual evoked potentials in chronic schizophrenic patients. Acta Psychiatr Scand 73(5):566–573. https://doi.org/10.1111/j.1600-0447.1986.tb02726.x
Sklar AL, Coffman BA, Salisbury DF (2020) Localization of early-stage visual processing deficits at schizophrenia spectrum illness onset using magnetoencephalography. Schizophr Bull 46(4):955–963. https://doi.org/10.1093/schbul/sbaa010
Yeap S, Kelly SP, Sehatpour P, Magno E, Garavan H, Thakore JH et al (2008) Visual sensory processing deficits in Schizophrenia and their relationship to disease state. Eur Arch Psychiatry Clin Neurosci 258(5):305–316. https://doi.org/10.1007/s00406-008-0802-2
McCleery A, Wynn JK, Lee J, Reavis EA, Ventura J, Subotnik KL et al (2020) Early visual processing is associated with social cognitive performance in recent-onset schizophrenia. Front Psychiatry 11:823. https://doi.org/10.3389/fpsyt.2020.00823
Herrera SN, Zemon V, Revheim N, Silipo G, Gordon J, Butler PD (2021) Cognitive function mediates the relationship between visual contrast sensitivity and functional outcome in schizophrenia. J Psychiatr Res 144:138–145. https://doi.org/10.1016/j.jpsychires.2021.09.055
Butler PD, Abeles IY, Weiskopf NG, Tambini A, Jalbrzikowski M, Legatt ME et al (2009) Sensory contributions to impaired emotion processing in schizophrenia. Schizophr Bull 35(6):1095–1107. https://doi.org/10.1093/schbul/sbp109
Zemon V, Kaplan E, Ratliff F (1980) Bicuculline enhances a negative component and diminishes a positive component of the visual evoked cortical potential in the cat. Proc Natl Acad Sci USA 77(12):7476–7478
Zemon V, Kaplan E, Ratliff F (1986) The role of GABA-mediated intracortical inhibition in the generation of visual evoked potentials. In: Cracco R, Bodis-Wollner I (eds) Evoked potentials, frontiers of clinical neuroscience. Alan R. Liss, Inc., New York, pp 287–295
Creutzfeldt OD, Kuhnt U (1973) Electrophysiology and topographical distribution of visual evoked potentials in animals. In: Jung R (ed) Handbook of sensory physiology 7/3. Springer, Berlin, pp 595–646
Zemon V, Ratliff F (1984) Intermodulation components of the visual evoked potential: responses to lateral and superimposed stimuli. Biol Cybern 50(6):401–408. https://doi.org/10.1007/BF00335197
Zemon V, Gordon J (2018) Quantification and statistical analysis of the transient visual evoked potential to a contrast-reversing pattern: a frequency-domain approach. Eur J Neurosci 48(2):1765–1788. https://doi.org/10.1111/ejn.14049
Zemon V, Ratliff F (1982) Visual evoked potentials: evidence for lateral interactions. Proc Natl Acad Sci USA 79(18):5723–5726. https://doi.org/10.1073/pnas.79.18.5723
Regan D (1989) Human brain electrophysiology: evoked potentials and evoked magnetic fields in science and medicine. Elsevier, New York
Zemon V, Gordon J (2006) Luminance-contrast mechanisms in humans: visual evoked potentials and a nonlinear model. Vision Res 46(24):4163–4180. https://doi.org/10.1016/j.visres.2006.07.007
Nakamura M, Kakigi R, Okusa T, Hoshiyama M, Watanabe K (2000) Effects of check size on pattern reversal visual evoked magnetic field and potential. Brain Res 872(1–2):77–86. https://doi.org/10.1016/s0006-8993(00)02455-0
Di Russo F, Pitzalis S, Spitoni G, Aprile T, Patria F, Spinelli D et al (2005) Identification of the neural sources of the pattern-reversal VEP. Neuroimage 24(3):874–886. https://doi.org/10.1016/j.neuroimage.2004.09.029
Shigeto H, Tobimatsu S, Yamamoto T, Kobayashi T, Kato M (1998) Visual evoked cortical magnetic responses to checkerboard pattern reversal stimulation: a study on the neural generators of N75, P100 and N145. J Neurol Sci 156(2):186–194
Ducati A, Fava E, Motti ED (1988) Neuronal generators of the visual evoked potentials: intracerebral recording in awake humans. Electroencephalogr Clin Neurophysiol 71(2):89–99
Schroeder C, Tenke C, Givre S, Arezzo J, Vaughan H (1991) Striate cortical contribution to the surface-recorded pattern-reversal VEP in the alert monkey. Vision Res 31(7):1143–1157
Kraut MA, Arezzo JC, Vaughan HG (1990) Inhibitory processes in the flash evoked potential of the monkey. Electroencephalogr Clin Neurophysiol 76(5):440–452
Noachtar S, Hashimoto T, Lüders H (1993) Pattern visual evoked potentials recorded from human occipital cortex with chronic subdural electrodes. Electroencephalogr Clin Neurophysiol 88(6):435–446. https://doi.org/10.1016/0168-5597(93)90032-k
Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC (2012) Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol Psychiatry 71(6):521–529. https://doi.org/10.1016/j.biopsych.2011.10.037
Lalor EC, Yeap S, Reilly RB, Pearlmutter BA, Foxe JJ (2008) Dissecting the cellular contributions to early visual sensory processing deficits in schizophrenia using the VESPA evoked response. Schizophr Res 98(1–3):256–264. https://doi.org/10.1016/j.schres.2007.09.037
Tzelepi A, Bezerianos T, Bodis-Wollner I (2000) Functional properties of sub-bands of oscillatory brain waves to pattern visual stimulation in man. Clin Neurophysiol 111(2):259–269. https://doi.org/10.1016/S1388-2457(99)00248-5
Porcaro C, Ostwald D, Hadjipapas A, Barnes GR, Bagshaw AP (2011) The relationship between the visual evoked potential and the gamma band investigated by blind and semi-blind methods. Neuroimage 56(3):1059–1071. https://doi.org/10.1016/j.neuroimage.2011.03.008
Carozzo S, De Carli F, Beelke M, Saturno M, Garbarino S, Martello C et al (2004) Factor structure of the human gamma band oscillatory response to visual (contrast) stimulation. Clin Neurophysiol 115(7):1669–1676. https://doi.org/10.1016/j.clinph.2004.02.025
First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS (1997) Structured clinical interview for DSM-IV axis II personality disorders. American Psychiatric Association, Washington, DC
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13(2):261–276. https://doi.org/10.1093/schbul/13.2.261
Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR (2005) What does the PANSS mean? Schizophr Res 79(2–3):231–238. https://doi.org/10.1016/j.schres.2005.04.008
Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Mizota A et al (2016) ISCEV standard for clinical visual evoked potentials (2016 update). Doc Ophthalmol 133(1):1–9
Klem GH, Luders HO, Jasper HH, Elger C (1999) The ten-twenty electrode system of the International Federation: The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol 52:3–6
Zemon V, Eisner W, Gordon J, Grose-Fifer J, Tenedios F, Shoup H (1995) Contrast-dependent responses in the human visual system: childhood through adulthood. Int J Neurosci 80(1–4):181–201
Butler PD, Hoptman MJ, Nierenberg J, Foxe JJ, Javitt DC, Lim KO (2006) Visual white matter integrity in schizophrenia. Am J Psychiatry 163(11):2011–2013. https://doi.org/10.1176/ajp.2006.163.11.2011
Kaplan E (2004) P, and K pathways of the primate visual system. In: Chalupa LM, Werner JS (eds) The visual neurosciences, vol 1. MIT Press, Cambridge, pp 481–493
Keri S, Kiss I, Kelemen O, Benedek G, Janka Z (2005) Anomalous visual experiences, negative symptoms, perceptual organization and the magnocellular pathway in schizophrenia: a shared construct? Psychol Med 35(10):1445–1455. https://doi.org/10.1017/S0033291705005398
Coyle JT (2012) NMDA receptor and schizophrenia: a brief history. Schizophr Bull 38(5):920–926. https://doi.org/10.1093/schbul/sbs076
Mast J, Victor JD (1991) Fluctuations of steady-state VEPs: interaction of driven evoked potentials and the EEG. Electroencephalogr Clin Neurophysiol 78(5):389–401
Johnson CA, Casson EJ (1995) Effects of luminance, contrast, and blur on visual acuity. Optom Vis Sci 72(12):864–869
Sokol S, Moskowitz A (1981) Effect of retinal blur on the peak latency of the pattern evoked potential. Vision Res 21(8):1279–1286. https://doi.org/10.1016/0042-6989(81)90232-7
Acknowledgements
We thank Gail Silipo for her tireless work as the coordinator for this study, and we are grateful to all those who participated in this study. We acknowledge funding from the National Institute of Mental Health (R43MH083364).
Author information
Authors and Affiliations
Contributions
Y-TT, VZ, JG, and PB designed the study, analyzed the data, interpreted the results, and wrote the manuscript. PB supervised VEP data collection.
Corresponding author
Ethics declarations
Conflict of interest
Zemon is a principal in and Gordon is a consultant for VeriSci Corp., a company that manufactured the Neucodia system used for the experiments conducted in this study. These two authors are shareholders in this company, which has a licensing agreement with Konan Medical USA. Tsai and Butler have no interests to declare.
Ethics approval
The protocol for this study was approved by the Institutional Review Board of the Nathan Kline Institute for Psychiatric Research and Rockland Psychiatric Center.
Informed consent
All participants gave their written informed consent.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statement on the welfare of animals
There were no animals involved in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsai, YT., Gordon, J., Butler, P. et al. Frequency-domain analysis of transient visual evoked potentials in schizophrenia. Doc Ophthalmol 146, 211–227 (2023). https://doi.org/10.1007/s10633-023-09921-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-023-09921-2