Abstract
Background
Ozanimod showed efficacy and safety in the phase 2 STEPSTONE study conducted in patients with moderately to severely active Crohn’s disease.
Aims
This analysis assessed the effects of ozanimod on circulating lymphocytes in Crohn’s disease.
Methods
Patients received ozanimod 0.92 mg for 12 weeks. Lymphocyte subtypes were evaluated using multicolor flow analysis on blood samples collected before treatment and on Week 12. Absolute lymphocyte count changes were analyzed by Wilcoxon signed rank tests. Disease activity changes and efficacy outcomes were evaluated at Week 12, and associations with lymphocyte subtype levels were assessed using Spearman’s correlation and logistic regression.
Results
Reductions in median total T, Th, and cytotoxic T cells occurred at Week 12 (45.4%–76.8%), with reductions in most subtypes of 47.5% to 91.3% (P < 0.001). CD8+ terminally differentiated effector memory cells were largely unaffected (median change, − 19%; P = 0.44). Reductions in median total B cells occurred at Week 12 (76.7%), with reductions in subtypes of 71.4% to 81.7% (P < 0.001). Natural killer and monocyte cell counts were unchanged. Greater baseline levels and changes in nonswitched memory B cells were significantly associated with clinical, endoscopic, and histologic efficacy (P < 0.05, all comparisons).
Conclusions
Ozanimod reduced circulating levels of all B-cell and most T-cell subsets but not monocytes or natural killer cells. Key subsets relevant to immune surveillance were not reduced, supporting the low risk of infection and malignancy with ozanimod in chronic inflammatory diseases. Levels of nonswitched memory B cells were associated with efficacy, providing a potential marker for ozanimod response.
Trial Registration
ClinicalTrials.gov: NCT02531113, EudraCT: 2015–002025–19
Graphical Abstract

Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD), which comprises Crohn’s disease (CD) and ulcerative colitis (UC), affects more than 6.8 million individuals globally [1]. Substantial increases in the incidences of both diseases have been noted over the past 2 decades in newly industrialized countries [1, 2]. Although the etiology of IBD remains largely unknown, studies indicate that IBD is characterized by a pathological immune response to the gut microbiota in the context of both genetic and environmental risk factors [3]. Upon entering the intestinal compartment, dendritic cells respond to microbial antigens and subsequently migrate to local lymphoid tissues where they present these antigens to naive T cells [4,5,6]. Consequently, these cells become activated and differentiate under the influence of cytokines into specific helper T (Th) subtypes, which then migrate out of the lymph nodes and return by way of the lymphatics into the circulation [4,5,6]. This process facilitates the amplification of cellular immune response by allowing effector T cells to return to inflamed tissues [4, 5]. In IBD, the migration of lymphocytes into the intestinal mucosa is critical to the development of chronic inflammation [4]. Other types of lymphocytes, such as B cells and natural killer (NK) cells, are also involved in the pathogenesis of IBD [3, 6]. Accordingly, blocking the egress of lymphocytes from lymphoid tissues into the bloodstream is an established therapeutic approach with proven efficacy and safety in IBD [7].
Sphingosine 1-phosphate (S1P) is a phospholipid cell membrane component with a role in lymphocyte trafficking and can promote inflammation [5, 8]. S1P levels are increased in inflamed tissues, which exacerbates inflammatory processes due to recruitment of immune cells and inflammatory mediators [5, 8]. S1P levels are higher in circulation than in lymphoid tissues, creating a differential concentration gradient [5, 8,9,10]. S1P1 receptors expressed on lymphocytes follow this gradient, guiding lymphocytes toward the higher S1P concentration in circulation [5, 8,9,10]. Interference with this process via S1P1 receptor modulation (leading to receptor internalization) blocks the capacity of lymphocytes to egress from lymphoid tissues, thereby reducing the number of some lymphocyte subsets in circulation, with preservation of immunosurveillance [5, 9,10,11]. Ozanimod, an S1P receptor modulator that selectively targets S1P1 and S1P5 receptors [10], is approved in the United States, European Union, and other countries for the treatment of adults with moderately to severely active UC and relapsing multiple sclerosis (MS) [12, 13].
The effect of ozanimod in patients with moderately to severely active CD was evaluated in the phase 2 STEPSTONE study (NCT02531113), a multicenter, uncontrolled, 12-week trial [14]. Treatment with ozanimod resulted in clinical, endoscopic, and histologic improvements after 12 weeks of treatment. A mean reduction in the Simple Endoscopic Score for Crohn’s Disease (SES-CD; primary endpoint) of 2.2 points was observed at Week 12. Ozanimod was generally well tolerated. The most commonly reported treatment-emergent adverse events (TEAEs) besides CD flare (26%) were abdominal pain (15%), lymphopenia (13%), arthralgia (13%), and nausea (12%), and 16% of patients discontinued treatment due to TEAEs. The most common serious TEAE besides CD flare (9%) was abdominal abscess (3%). There were no clinically important changes in heart rate at treatment initiation. Confirmatory phase 3 studies are underway in the YELLOWSTONE clinical trials (induction studies: NCT03440372, NCT03440385; maintenance study: NCT03464097; open-label extension [OLE]: NCT03467958).
Although the efficacy of ozanimod has been demonstrated in patients with UC and MS [15,16,17], important questions remain regarding its mechanism of action. Specifically, despite the marked reductions in peripheral blood lymphocyte counts observed following treatment, ozanimod does not result in a substantial increase in risk of infection [15,16,17]. This observation is consistent with the notion of a relatively selective immunosuppressive action. Here, using an exploratory analysis of data from the STEPSTONE study, the effects of ozanimod on circulating lymphocytes, including T-cell and B-cell subtypes, were further assessed in patients with moderately to severely active CD. The association of circulating lymphocytes with ozanimod efficacy was also evaluated in this analysis.
Methods
Study Design
The design of STEPSTONE (NCT02531113, EudraCT: 2015–002025–19) has been previously described [14]. Briefly, STEPSTONE is a 12-week, multicenter, uncontrolled, prospective observer-blinded induction study conducted in 28 hospitals and community centers across the United States, Canada, Hungary, Poland, and Ukraine. All enrolled patients received ozanimod 0.92 mg (equivalent to ozanimod HCl 1 mg) daily for approximately 12 weeks, including an initial 7-day dose-escalation period consisting of 4 days of ozanimod 0.23 mg (equivalent to ozanimod HCl 0.25 mg) daily and 3 days of ozanimod 0.46 mg (equivalent to ozanimod HCl 0.5 mg) daily. The final dose of ozanimod 0.92 mg (equivalent to ozanimod HCl 1 mg) daily was reached on Day 8. All patients who completed the 12-week induction period were eligible to enter the 100-week extension period of the study.
Patients
Adults aged 18 to 75 years with a confirmed diagnosis of CD by clinical endoscopic evidence and corroborated by histology at least 2 months before screening were enrolled. Eligible patients were also required to meet the following criteria: a Crohn’s Disease Activity Index (CDAI) score of 220 to 450; an SES-CD of at least 6 (or in isolated ileitis SES-CD ≥ 4); an average daily stool score of at least 4 points and/or an average daily abdominal pain score of at least 2 points. Patients also had a prior inadequate response, loss of response, or intolerance to 5-aminosalicylates, corticosteroids, immunomodulators, or biologic therapies (i.e, an anti–tumor necrosis factor or anti-integrin at an approved dose). The following background therapies for CD were allowed during the study through at least Week 8: oral aminosalicylates at a stable dose for ≥ 3 weeks prior to screening endoscopy, prednisone (≤ 20 mg/day) or equivalent at a stable dose (≤ 9 mg/day) for ≥ 2 weeks prior to screening endoscopy, or budesonide at a stable dose for ≥ 2 weeks prior to screening endoscopy. Key exclusion criteria included diagnosis of UC, indeterminate colitis, or CD isolated to the stomach, duodenum, jejunum, or perianal region, without colonic or ileal involvement.
This study was conducted in accordance with the Declaration of Helsinki, the Good Clinical Practice Guideline established by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, and applicable drug and data protection laws and regulations of the countries where the trial was conducted. Protocols, amendments, and documentation of informed consent were reviewed and approved by institutional review boards or independent ethics committee for each study center prior to initiation of the study. All enrolled patients provided written informed consent before entering the study.
Flow Cytometry Analysis
Flow cytometry was performed by Q2 Solutions (Durham, NC, USA). A total of 48 lymphocyte subtypes were evaluated using multicolor flow analysis using a Becton Dickinson FACSCanto II (BD Biosciences, Franklin Lakes, NJ, USA). Blood samples were shipped ambient and tested in real time. Analysis was performed on blood samples from the enrolled patients collected on Day 1 (prior to treatment) and at Week 12. An A167 Q-TBNK assay (Quintiles Laboratories) evaluated the following six subtypes: total T cells (CD3+), Th cells (CD3+CD4+), cytotoxic T (Tc) cells (CD3+CD8+), B cells (CD3−CD19+), NK cells (CD3−CD56+/CD16+), and monocytes (CD14+). A V176 B-cell assay (Quintiles Laboratories) evaluated seven subtypes: B cells (CD45+CD19+), memory B cells (CD19+IgD−CD27+), double-negative memory B cells (CD19+IgD−CD27−), nonswitched memory B cells (CD19+IgD+CD27+), naive B cells (CD19+IgD+CD27−), and plasmablasts (CD19+CD20−IgD-CD27+CD38hi). A V180 T-cell assay (Quintiles Laboratories) examined 11 T-cell subtypes: CD4+ naive T cells (CD4+CD45RA+CD197+), Th1 cells (CD4+CD45RA−CD183+CD196−), Th1/Th17 cells (CD4+CD45RA−CD183+CD196+), Th2 cells (CD4+CD45RA−CD183−CD196−), Th17 cells (CD4+CD45RA−CD183−CD196+), CD4+ effector memory (EM) T cells (CD4+CD45RA−CD197−), CD4+ central memory (CM) T cells (CD4+CD45RA−CD197+), CD8+ terminally differentiated EM (TEMRA) T cells (CD8+CD45RA+CD197−), CD8+ naive T cells (CD8+CD45RA+CD197+), CD8+ EM T cells (CD8+CD45RA−CD197−), and CD8+ CM T cells (CD8+CD45RA−CD197+). Information on the flow cytometry antibodies used in these assays is included in the Supplementary Methods.
Flow data were initially expressed as percentage of gated events represented by each cell population, as indicated by the percentage in the reported unit, or description of subtypes of lymphocytes. Absolute cell counts were generated and reported in units of cells per microliter. Total lymphocyte counts for lymphocytes plus monocytes were determined based on staining with CD45 and side scatter. Total T-cell, total Th, and Tc populations were identified based on positive staining with CD3 and side scatter, CD4, and CD8, respectively.
Disease Activity and Efficacy Analysis
Disease activity measures were assessed at baseline (on Day 1 before treatment) and Week 12, and change from baseline to Week 12 was determined for SES-CD, size of ulcers, extent of ulcerated surface, extent of affected surface, presence of narrowings, CDAI, Robart’s Histopathology Index (RHI), Geboes Histology Activity Score (GHAS), and stool frequency. Efficacy outcomes were assessed at Week 12 and included clinical response (CDAI reduction from baseline of ≥ 100 points), clinical remission (CDAI score of < 150), endoscopic response–25 (≥ 25% decrease in SES-CD from baseline), endoscopic response–50 (≥ 50% decrease in SES-CD from baseline), endoscopic remission (SES-CD ≤ 4 points and SES-CD decrease ≥ 2 points with no SES-CD subscore > 1 point), global GHAS remission (no active inflammation in any measured segment based on the GHAS), RHI remission (no active inflammation in any measured segment based on the RHI), and RHI mucosal healing (endoscopic remission plus RHI remission).
Statistical Analysis
Bristol Myers Squibb (Princeton, NJ, USA) and Labcorp Drug Development (formerly Covance; Princeton, NJ, USA) provided statistical analysis. Data for the flow cytometry analyses were summarized using descriptive statistics. All comparisons between cell counts on Week 12 and Day 1 were analyzed by the Wilcoxon signed rank test using SAS version 9.3 or higher (SAS Institute, Cary, NC, USA). Change in absolute cell count was calculated as absolute cell counts on Week 12 minus those on Day 1, and the percentage change in absolute cell count was computed as 100 × (absolute counts on Week 12 – Day 1)/absolute cell count on Day 1 for each patient with test values from Day 1 and Week 12.
Spearman’s correlation was used to analyze associations between change from baseline to Week 12 in disease activity measures and (1) baseline levels of lymphocyte subtypes and (2) change from baseline to Week 12 in lymphocyte subtypes. Logistic regression was used to analyze associations between Week 12 efficacy outcomes and baseline levels of lymphocyte subtypes. Changes from baseline and percentage changes from baseline of flow cytometry measurements between Week 12 and baseline were calculated for those with the data from both time points, and no imputation was made to project or assign values to missing values in CDAI, SES-CD, or flow cytometry data.
Results
Patients
Sixty-nine patients were enrolled between November 17, 2015, and August 18, 2016. Demographics and baseline characteristics have been previously described [14]. Briefly, the patients’ mean age was 37.7 years, 52% were women, and 90% had a history of previous corticosteroid use.
Of the 69 patients who received treatment, 58 (84%) completed the 12-week treatment period. The present flow cytometry analyses included 67 patients with available data; depending on the statistical model used, different sample sizes were included in the presented results. Among the 69 enrolled patients, 54 had CDAI and SES-CD results at baseline and Week 12, but two of the 54 patients did not have any flow cytometry data. As a result, only 52 patients completed the 12-week induction and had clinical outcomes and flow data for baseline and Week 12 for the Spearman’s correlation and logistic regression analyses.
Effect of Ozanimod on Peripheral Blood Mononuclear Subsets
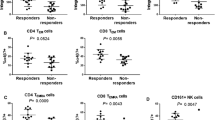
Following 12 weeks of treatment with ozanimod, statistically significant (P < 0.0001) reductions in median absolute cell counts of total T cells (CD3+), Th cells (CD3+CD4+), Tc cells (CD3+CD8+), and B cells (CD3−CD19+) were observed compared with absolute cell counts at Day 1 (Fig. 1A). Reductions in median absolute cell counts ranged from 45.4 to 76.8%, with the greatest reductions noted for Th cells and B cells, followed by total T cells and Tc cells (Fig. 1B). Median absolute cell counts of NK cells (CD3−CD56+/CD16+) and monocytes (CD14+) were maintained from Day 1 to Week 12 (P = 0.64 and P = 0.72, respectively) (Fig. 1A). Changes in median absolute cell counts of NK cells and monocytes were minimal, with nonsignificant changes of 4.1% and − 1.3%, respectively (Fig. 1B).
Median absolute cell counts (cell/μL) at A Day 1 and Week 12 and B percentage changes at Week 12 from Day 1 in median absolute cell count of peripheral blood mononuclear subsets. In panel B, the solid rectangles span the first and third quartiles, the median is indicated by the white line inside each rectangle, and the green dots represent outliers. NK, natural killer; Tc, cytotoxic T; Th, helper T
Effect of Ozanimod on B and T Lymphocytes
Significant reductions (P < 0.0001) in median absolute B-cell counts, including all B cells (CD45+CD19+), naive B cells (CD19+IgD+CD27−), memory B cells (CD19+IgD−CD27+), double-negative memory B cells (CD19+IgD−CD27−), nonswitched memory B cells (CD19+IgD+CD27+), and plasmablasts (CD19+CD20−IgD−CD27+CD38hi), at Week 12 were observed (Fig. 2A), with reductions in median absolute cell counts ranging from 71.4 to 81.7% (Fig. 2B).
Median absolute cell counts (cell/μL) at A Day 1 and Week 12 and B percentage changes at Week 12 from Day 1 in median absolute cell count of B lymphocytes. In panel B, the solid rectangles span the first and third quartiles, the median is indicated by the white line inside each rectangle, and the green dots represent outliers
In the T-cell populations measured, statistically significant reductions (P < 0.0001) were observed in CD8+ EM (CD8+CD45RA−CD197−), Th1 (CD4+CD45RA−CD183+CD196−), Th2 (CD4+CD45RA−CD183−CD196−), CD4+ EM (CD4+CD45RA−CD197−), CD8+ CM (CD8+CD45RA−CD197+), Th1/Th17 (CD4+CD45RA−CD183+CD196+), CD4+ CM (CD4+CD45RA−CD197+), Th17 (CD4+CD45RA−CD183−CD196+), CD4+ naive (CD4+CD45RA+CD197+), and CD8+ naive (CD8+CD45RA+CD197+) T cells at Week 12 (Fig. 3A), with reductions in median absolute cell counts from Day 1 ranging from 47.5% to 91.3% (Fig. 3B). However, median absolute cell counts for CD8+ TEMRA T cells (CD8+CD45RA+CD197−) were largely unaffected from Day 1 to Week 12 (P = 0.44) (Fig. 3A), with a reduction in median absolute cell counts of 19% (Fig. 3B).
Median absolute cell counts (cell/μL) at A Day 1 and Week 12 and B percentage changes at Week 12 from Day 1 in median absolute cell count of T lymphocytes. In panel B, the solid rectangles span the first and third quartiles, the median is indicated by the white line inside each rectangle, and the green dots represent outliers. CM, central memory; EM, effector memory; TEMRA, terminally differentiated effector memory; Th, helper T
Association of Baseline Lymphocyte Subtype Levels with Changes in Disease Activity and Week 12 Efficacy Outcomes
Multiple positive and negative correlations relative to disease activity scores were shown with T and B cells (Supplementary Figs. 1 and 2); however, nonswitched memory B cells had the most consistent correlations across disease activity endpoints. Notably, baseline levels of nonswitched memory B cells were significantly correlated with change in extent of affected surface (Spearman’s rho = – 0.44; P < 0.05), change in RHI (Spearman’s rho = – 0.34; P < 0.05), and change in GHAS (Spearman’s rho = – 0.47; P < 0.05).
Most cell types were associated with 1 or 2 clinical outcomes (Supplementary Table 1). However, nonswitched memory B cells were significantly associated with most of the efficacy endpoints, including endoscopic response–25, endoscopic response–50, endoscopic remission, global GHAS remission, and RHI mucosal healing (P < 0.05).
Two positive correlations between baseline levels of lymphocyte subtypes were observed (Supplementary Fig. 3). Baseline levels of the CD4+ EM T-cell (CD49d + B7 +) type and the CD8+ skin-homing CCR4 EM cell type were significantly correlated (Spearman’s rho = 0.48; P < 0.05). Baseline levels of the IgD CD27 memory B-cell type and the nonswitched memory B-cell type were significantly correlated (Spearman’s rho = 0.57; P < 0.05).
Association of Changes in Lymphocyte Subtype Levels with Changes in Disease Activity
Positive and negative correlations relative to disease activity measures were shown with changes in T cells, B cells, and monocytes (Fig. 4 and Supplementary Fig. 4). Notably, changes in levels of nonswitched memory B cells were significantly correlated with change in CDAI (Spearman’s rho = 0.38; P < 0.05), change in RHI (Spearman’s rho = 0.37; P < 0.05), change in GHAS (Spearman’s rho = 0.43, P < 0.05), and change in stool frequency (Spearman’s rho = 0.43; P < 0.05). There were no significant correlations between changes in levels of lymphocyte subtypes (Supplementary Fig. 5).
Association of change from baseline to Week 12 in levels of lymphocyte subtypes with change from baseline to Week 12 in disease activity measures. Spearman’s rho is represented by the values inside each square. Squares without an “NS” are significant (P < 0.05). aExpressed in cells/µL. CDAI, Crohn’s Disease Activity Index; GHAS, Geboes Histology Activity Score; NS, not significant; RHI, Robart’s Histopathology Index; SES-CD, Simple Endoscopic Score for Crohn’s Disease; TEMRA, terminally differentiated effector memory
Discussion
This multiparametric cell flow cytometry analysis is the first to assess the effect of ozanimod therapy on lymphocyte subtypes in patients with moderately to severely active CD. Overall, 12 weeks of ozanimod treatment resulted in reductions in most lymphocyte subtypes, including total T-cell, Th, and Tc counts and all B-cell subtypes. In contrast, NK cells, monocytes, and some specific T-cell subtypes comprising CD8+ TEMRA T cells were largely unaffected. Additionally, baseline levels of and changes in nonswitched memory B cells were significantly associated with clinical, endoscopic, and histologic responses to ozanimod.
As reported previously [11], these findings are consistent with the expected effects of S1P1 receptor modulation, considering that expression of C–C motif chemokine receptor 7, linked to S1P1 receptor–mediated egress of lymphocytes from lymph nodes, is expressed on B cells and naive and CM T cells, but not on EM T cells and CD8+ TEMRA T cells [18]. Moreover, cell flow cytometry data from this analysis in patients with moderately to severely active CD are consistent with findings in patients with relapsing forms of MS [11] as well as in healthy controls receiving ozanimod [19]. The present findings are further supported by data for other S1P modulators in MS, namely fingolimod [18], siponimod [20], and ponesimod [21, 22], all of which have shown similar effects on the circulating lymphocyte profile.
The mechanism of action of vedolizumab, an approved therapy for moderately to severely active CD, also targets lymphocytes [23, 24]. Vedolizumab binds to the α4β7 integrin, which blocks the interaction of α4β7 integrin with mucosal addressin cell adhesion molecule-1. Based on its mechanism of action, vedolizumab does not impact peripheral lymphocyte levels, in contrast to the effects of ozanimod. Instead, vedolizumab inhibits T-cell migration into inflamed gastrointestinal tissue. The focus of this analysis was on the impact of ozanimod on lymphocyte subsets in circulation in patients with CD, which is directly related to the mechanism of action of ozanimod. Therefore, this analysis lacks data on tissue lymphocyte populations, so comparisons to vedolizumab cannot be made. Tissue lymphocyte levels, along with the relationship to treatment response with ozanimod, will be explored in future analyses of CD.
The differential decreases in circulating lymphocytes observed in the present analysis indicate that, despite overall reductions in T- and B-lymphocyte subtypes that are involved in adaptive immunity and relevant to the pathophysiology of CD, key subtypes, including NK cells and monocytes, are not significantly reduced with ozanimod treatment. NK cells and monocytes are involved with immune surveillance against infections and tumors [25,26,27]. Neutrophils are also involved with immune surveillance [28]. While this analysis did not assess neutrophil levels in response to ozanimod treatment in patients with CD, previous analyses in patients with UC demonstrated that ozanimod decreased peripheral neutrophil levels, but not to levels below the normal range [29]. This relatively short 12-week study was not designed to assess preservation of immune surveillance, but our findings suggest that immune surveillance may remain relatively intact with ozanimod treatment, an observation that is clinically important and consistent with ozanimod safety results from clinical studies.
To date, clinical safety results demonstrate low risk of infection or malignancy in patients treated with ozanimod [14,15,16,17]. In the 52-week, randomized, double-blind, placebo-controlled True North phase 3 study of 1012 patients with moderately to severely active UC, incidences of serious infection (≤ 1.8%), herpes zoster (≤ 2.2%), and malignancy (≤ 0.4%) were low in the ozanimod groups and similar to those of the placebo groups [17]. In an interim analysis of the True North OLE study of long-term ozanimod treatment in UC (1201 total patient-years of exposure; data cutoff: September 30, 2020), exposure-adjusted incidence rates of serious infection, herpes zoster, and malignancy were 1.5, 1.7, and 0.5 per 100 patient-years, respectively [30]. In the uncontrolled STEPSTONE study of 69 patients with CD, 5 (7%) patients reported TEAEs of upper respiratory tract infection and 5 (7%) reported urinary tract infection [14]. Serious TEAEs included Campylobacter infection, sepsis, and pancreatic carcinoma (n = 1 each) [14]. While the safety of ozanimod in CD is being further evaluated in the phase 3 YELLOWSTONE clinical studies, exposure-adjusted incidence rates of serious infection, herpes zoster, and malignancy with ozanimod in patients with UC were similar to or lower than those with other advanced therapies (ie, adalimumab, vedolizumab, ustekinumab, and upadacitinib) in patients with UC or CD [31,32,33,34,35,36].
Similar to findings in UC, the incidence of infection-related TEAEs was similar between the ozanimod and interferon beta-1a groups, serious infections, herpes zoster, and malignancies were infrequent, and no serious opportunistic infections were reported in patients treated with ozanimod for at least 12 months in randomized, double-blind, active-controlled phase 3 studies of relapsing MS [15, 16]. In an interim analysis of the DAYBREAK OLE study of long-term ozanimod treatment in MS (12,617 total patient-years of exposure; data cutoff: 2 February 2021), exposure-adjusted incidence rates of serious infection, herpes zoster, and malignancy were 0.7, 0.5, and 0.3 per 100 patient-years, respectively [37]. Additionally, an analysis of patients with MS treated with ozanimod 0.92 mg in the phase 3 SUNBEAM and RADIANCE parent studies and the DAYBREAK OLE study showed that absolute lymphocyte count levels were not associated with serious infections or opportunistic infections [38].
Changes in the counts of specific lymphocyte subsets have been previously evaluated as surrogate markers of efficacy in antiretroviral therapy [39], are important as a pharmacodynamic marker for ozanimod, and provide a better understanding of the mechanism of action of ozanimod. Dysregulation of immune response plays a critical role in the pathogenesis of IBD, and B cells are among the immune cell types that are involved in intestinal inflammation [3, 6]. Nonswitched memory B cells, which are involved in T cell–independent immune responses, have been implicated in some inflammatory and immune-mediated diseases [40,41,42]. Our analysis demonstrated that higher levels of nonswitched memory B cells at baseline were associated with better endoscopic and histologic efficacy outcomes at Week 12. Additionally, changes in levels of nonswitched memory B cells after 12 weeks of treatment were positively correlated with changes in clinical and histologic disease activity after 12 weeks of treatment, indicating that decreases in levels of this lymphocyte subtype are associated with decreases in disease activity with ozanimod.
The limitations of this analysis include the lack of a control group given the uncontrolled study design and the lack of follow-up time points beyond 12 weeks to assess whether changes in lymphocyte subtypes stabilize over time. Also, in the absence of larger datasets and validation in other patient populations, our analysis does not provide adequate data to definitively demonstrate the predictive value of nonswitched memory B cells for ozanimod response. However, these concepts will be explored in future analyses. While the assessment of nonswitched memory B cells as a biomarker is likely feasible at academic institutions, future analyses along with simplified assays are needed to expand feasibility to other clinical settings and implement use in routine clinical practice. Additionally, the applicability of specific lymphocyte subsets as surrogate markers for specific safety outcomes in CD was not assessed. However, as discussed, prior analyses have demonstrated a low risk of infections and malignancies with ozanimod [14,15,16,17], and lymphocyte levels were not associated with serious or opportunistic infections in patients receiving ozanimod [38].
Although our results demonstrate the effect of ozanimod on peripheral lymphocyte subsets in patients with CD, additional analyses for further characterization of the effects of ozanimod on lymphocyte subtypes are still needed. Our study does not include a separate analysis of Th subsets in the CM and EM compartments. Additionally, gut-homing T cells and B cells need to be further analyzed with ozanimod treatment, as the gut-homing cell counts in our analysis were too small, due to sample limitations, to draw conclusions.
Ozanimod treatment resulted in consistent reduction of circulating levels of B-cell subsets but had a differential impact on T-cell subset reduction and did not alter monocytes or NK cells. This is consistent with previous findings in healthy volunteers and patients with MS. Despite overall reductions in lymphocyte subsets relevant to the pathophysiology of CD, key subsets involved with immune surveillance are not significantly reduced with ozanimod treatment, and immune surveillance may remain intact. These findings generally support clinical safety results showing low incidence of infection or malignancy in patients with CD, UC, or relapsing MS. While changes in most cell types were pharmacodynamic effects of ozanimod but not predictive of treatment response, nonswitched memory B-cell levels were associated with efficacy as measured by clinical, endoscopic, and histologic outcomes, implicating this lymphocyte subtype as a potential marker for ozanimod response in CD.
Data Availability
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html
References
GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30.
Ng SC, Shi HY, Hamidi N et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778.
de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27.
Arseneau KO, Cominelli F. Targeting leukocyte trafficking for the treatment of inflammatory bowel disease. Clin Pharmacol Ther. 2015;97:22–28.
Danese S, Furfaro F, Vetrano S. Targeting S1P in inflammatory bowel disease: new avenues for modulating intestinal leukocyte migration. J Crohns Colitis. 2018;12:S678–S686.
Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019:7247238.
Bencardino S, D’Amico F, Faggiani I et al. Efficacy and safety of S1P1 receptor modulator drugs for patients with moderate-to-severe ulcerative colitis. J Clin Med. 2023;12:5014.
Aoki M, Aoki H, Ramanathan R et al. Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediators Inflamm. 2016;2016:8606878.
Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301.
Scott FL, Clemons B, Brooks J et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173:1778–1792.
Harris S, Tran JQ, Southworth H et al. Effect of the sphingosine-1-phosphate receptor modulator ozanimod on leukocyte subtypes in relapsing MS. Neurol Neuroimmunol Neuroinflamm. 2020;7:e839.
Lamb YN. Ozanimod: first approval. Drugs. 2020;80:841–848.
Choi D, Stewart AP, Bhat S. Ozanimod: a first-in-class sphingosine 1-phosphate receptor modulator for the treatment of ulcerative colitis. Ann Pharmacother. 2022;56:592–599.
Feagan BG, Sandborn WJ, Danese S et al. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol. 2020;5:819–828.
Cohen JA, Comi G, Selmaj KW et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18:1021–1033.
Comi G, Kappos L, Selmaj KW et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18:1009–1020.
Sandborn WJ, Feagan BG, D’Haens G et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385:1280–1291.
Teniente-Serra A, Hervás JV, Quirant-Sánchez B et al. Baseline differences in minor lymphocyte subpopulations may predict response to fingolimod in relapsing-remitting multiple sclerosis patients. CNS Neurosci Ther. 2016;22:584–592.
Tran JQ, Hartung JP, Peach RJ et al. Results from the first-in-human study with ozanimod, a novel, selective sphingosine-1-phosphate receptor modulator. J Clin Pharmacol. 2017;57:988–996.
Gergely P, Nuesslein-Hildesheim B, Guerini D et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol. 2012;167:1035–1047.
D’Ambrosio D, Steinmann J, Brossard P et al. Differential effects of ponesimod, a selective S1P1 receptor modulator, on blood-circulating human T cell subpopulations. Immunopharmacol Immunotoxicol. 2015;37:103–109.
Jurcevic S, Juif PE, Hamid C et al. Effects of multiple-dose ponesimod, a selective S1P(1) receptor modulator, on lymphocyte subsets in healthy humans. Drug Des Devel Ther. 2017;11:123–131.
Entyvio [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; 2022.
Veny M, Garrido-Trigo A, Corraliza AM et al. Dissecting common and unique effects of anti-α4β7 and anti-tumor necrosis factor treatment in ulcerative colitis. J Crohns Colitis. 2021;15:441–452.
Kaur G, Trowsdale J, Fugger L. Natural killer cells and their receptors in multiple sclerosis. Brain. 2013;136:2657–2676.
Abel AM, Yang C, Thakar MS et al. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869.
Wolf BJ, Choi JE, Exley MA. Novel approaches to exploiting invariant NKT cells in cancer immunotherapy. Front Immunol. 2018;9:384.
Lehman HK, Segal BH. The role of neutrophils in host defense and disease. J Allergy Clin Immunol. 2020;145:1535–1544.
Harris S, Wu C, Li Y et al. The effect of ozanimod on circulating neutrophils: results from the True North study of patients with moderately to severely active ulcerative colitis [abstract MP054]. United Eur Gastroenterol J. 2022;10:227–228.
Wolf DC, Colombel JF, Ponich TP et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis [abstract Tu1458]. Gastroenterology. 2022;162:S969–S970.
Colombel JF, Sands BE, Rutgeerts P et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–851.
Colombel JF, Sandborn WJ, Reinisch W et al. Long-term safety of adalimumab in clinical trials in adult patients with Crohn’s disease or ulcerative colitis. Aliment Pharmacol Ther. 2018;47:219–228.
D’Haens G, Panés J, Louis E et al. Upadacitinib was efficacious and well-tolerated over 30 months in patients with Crohn’s disease in the CELEST extension study. Clin Gastroenterol Hepatol. 2022;20:2337-2346.e2333.
Ghosh S, Feagan BG, Ott E et al. Safety of ustekinumab in inflammatory bowel disease: pooled safety analysis through 5 years in Crohn’s disease and 4 years in ulcerative colitis. J Crohns Colitis. 2024. https://doi.org/10.1093/ecco-jcc/jjae013.
Wolf DC, Colombel JF, Ponich T, et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis [poster Tu1458]. Presented at DDW 2022, Digestive Disease Week; May 21–24, 2022; San Diego, CA, USA.
Loftus EV Jr, Feagan BG, Panaccione R et al. Long-term safety of vedolizumab for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52:1353–1365.
Cree BA, Selmaj KW, Steinman L et al. Long-term safety and efficacy of ozanimod in relapsing multiple sclerosis: up to 5 years of follow-up in the DAYBREAK open-label extension trial. Mult Scler. 2022;28:1944–1962.
Hartung HP, Steinman L, Bar-Or A et al. Relationship between infections and absolute lymphocyte count during phase 3 and open-label extension trials of ozanimod in patients with relapsing multiple sclerosis [abstract 707]. Mult Scler J. 2022;28(3S):622–623.
Bogner JR, Goebel FD. Lymphocyte subsets as surrogate markers in antiretroviral therapy. Infection. 1991;19:S103-108.
Meeuwsen JAL, van Duijvenvoorde A, Gohar A et al. High levels of (un)switched memory B cells are associated with better outcome in patients with advanced atherosclerotic disease. J Am Heart Assoc. 2017;6:e005747.
Simon D, Balogh P, Bognár A et al. Reduced non-switched memory B cell subsets cause imbalance in B cell repertoire in systemic sclerosis. Clin Exp Rheumatol. 2016;34:30–36.
Weller S, Braun MC, Tan BK et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654.
Acknowledgments
Editorial and writing assistance was provided by Anny Wu, PharmD, of Peloton Advantage, LLC, an OPEN Health company, and was funded by Bristol Myers Squibb. This manuscript, including related data, figures, and tables, has not been previously published and the manuscript is not under consideration elsewhere.
Funding
This work was supported by Bristol Myers Squibb.
Author information
Authors and Affiliations
Contributions
Conceptualization: Sarah Harris. Data curation: Sarah Harris, Brian G. Feagan, Stephen Hanauer, Severine Vermeire, Subrata Ghosh, Jim Yan, Chun Wu, Yanhua Hu, Rachel Maddux, Douglas C. Wolf, Geert D’Haens. Formal analysis: Sarah Harris, Brian G. Feagan, Stephen Hanauer, Severine Vermeire, Subrata Ghosh, Chun Wu, Yanhua Hu, Rachel Maddux, Douglas C. Wolf, Geert D’Haens. Funding acquisition: Not applicable. Investigation: Not applicable. Methodology: Sarah Harris. Project administration: Not applicable. Resources: Not applicable. Software: Not applicable. Supervision: Not applicable. Validation: Not applicable. Visualization: Not applicable. Writing – original draft: Not applicable. Writing – review & editing: Sarah Harris, Brian G. Feagan, Stephen Hanauer, Severine Vermeire, Subrata Ghosh, Jim Yan, Chun Wu, Yanhua Hu, Rachel Maddux, Douglas C. Wolf, Geert D’Haens.
Corresponding author
Ethics declarations
Conflicts of interest
Sarah Harris, Chun Wu, Yanhua Hu, and Rachel Maddux: employees and/or shareholders of Bristol Myers Squibb. Brian G. Feagan: consulted for AbbVie, ActoGeniX, Albireo, Amgen, AstraZeneca, Avaxia Biologics, Baxter, Biogen Idec, Boehringer Ingelheim, Bristol Myers Squibb, Calypso, Celgene, Elan, Eli Lilly, enGene, Ferring Pharmaceuticals, Roche/Genentech, gIcare Pharma, Gilead, Given Imaging, GlaxoSmithKline, Ironwood, Janssen, Johnson & Johnson, Lexicon, Merck, Millennium, Nektar, Novo Nordisk, Pfizer, Prometheus Laboratories, Protagonist, Sanofi, and UCB; and is a director at Robarts Clinical Trials. Stephen Hanauer: consulted for AbbVie, Actavis, Boehringer Ingelheim, Bristol Myers Squibb, Caremark, Celgene, Ferring Pharmaceuticals, Janssen, Merck, Pfizer, Salix, Sanofi-Aventis, Shire, and UCB. Severine Vermeire: received research funding from AbbVie, Galapagos, Johnson & Johnson, Pfizer, and Takeda; and has received consulting and/or speaking fees from AbbVie, Abivax, AbolerIS Pharma, AgomAb, Alimentiv, Arena, AstraZeneca, Avaxia, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, CVasThera, Cytoki Pharma, Dr Falk Pharma, Eli Lilly, Ferring, Galapagos, Genentech/Roche, Gilead, GlaxoSmithKline, Hospira, IMIDomics, Janssen, Johnson & Johnson, Materia Prima, MiroBio, Morphic Therapeutic, MRM Health, MSD, Mundipharma, Pfizer, ProDigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillotts Pharma AG, and Zealand. Subrata Ghosh: received research funding from AbbVie; has served as a speaker for AbbVie and MSD; and has consulted for AbbVie, Bristol Myers Squibb, Celgene, Janssen, Novo Nordisk, and Pfizer. Jim Yan: employee and shareholder of Laboratory Corporation of America Holdings. Douglas C. Wolf: received honoraria as a speaker, consultant, and/or advisory board member from AbbVie, Arena, Celgene/Bristol Myers Squibb, Janssen, Pfizer, Prometheus, Takeda, and UCB. Geert D’Haens: served as an advisor for AbbVie, Agomab Therapeutics, Alimentiv, Applied Molecular Therapeutics, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Cytoki, Eli Lilly, Exeliom Biosciences, Ferring, Galapagos, GlaxoSmithKline, Gossamer Bio, Immunic, Johnson & Johnson, Pfizer, Polpharma, ProciseDx, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Seres, Takeda, Tillotts, and Versant; and received speaker fees from AbbVie, Bristol Myers Squibb, Eli Lilly, Galapagos, Johnson & Johnson, Pfizer, Takeda, and Tillotts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Harris, S., Feagan, B.G., Hanauer, S. et al. Ozanimod Differentially Impacts Circulating Lymphocyte Subsets in Patients with Moderately to Severely Active Crohn’s Disease. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08391-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08391-z