Abstract
Background
Activation of the insulin-like growth factor 1 receptor (IGF-1R)-mediated Janus kinase (JAK)1/2-Stat3 pathway contributes to hepatocarcinogenesis. Specifically, a previous study showed that IGF-1R inhibition downregulated Midkine expression in hepatocellular carcinoma (HCC).
Aims
The present study investigated the role of IGF-1R-JAK1/2-Stat3 and Midkine signaling in HCC, in addition to the molecular link between the IGF-1R-Stat3 pathway and Midkine.
Methods
The expression levels of IGF-1R, Stat3, and Midkine were measured using reverse transcription-quantitative PCR, following which the association of IGF-1R with Stat3 and Midkine expression was evaluated in HCC. The molecular link between the IGF-1R-Stat3 pathway and Midkine was then investigated in vitro before the effect of IGF-1R-Stat3 and Midkine signaling on HCC growth and invasion was studied in vitro and in vivo.
Results
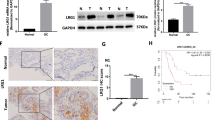
IGF-1R, Stat3, and Midkine mRNA overexpressions were all found in HCC, where the levels of Stat3 and Midkine mRNA correlated positively with those of IGF-1R. In addition, Midkine mRNA level also correlated positively with Stat3 mRNA expression in HCC tissues. IGF-1R promoted Stat3 activation, which in turn led to the upregulation of Midkine expression in Huh7 cells. Similarly, Midkine also promoted Stat3 activation through potentiating JAK1/2 phosphorylation. Persistent activation of this Stat3-Midkine-Stat3 positive feedback signal loop promoted HCC growth and invasion, the inhibition of which resulted in significant antitumor activities both in vitro and in vivo.
Conclusions
Constitutive activation of the IGF-1R-mediated Stat3-Midkine-Stat3 positive feedback loop is present in HCC, the inhibition of which can serve as a potential therapeutic intervention strategy for HCC.








Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- IGF-1:
-
Insulin-like growth factor 1
- IGF-1R:
-
Insulin-like growth factor 1 receptor
- JAK1/2:
-
Janus kinase ½
- MAPK:
-
Mitogen-activated protein kinase
- MANT:
-
Matched adjacent nontumorous tissues
- MTT:
-
Methyl thiazolyl tetrazolium
- NALT:
-
Normal adult liver tissues
- PI3K:
-
Phosphatidyl-inositol-3 kinase
- qChIP:
-
Quantitative chromatin immunoprecipitation
- PIAS:
-
Protein inhibitors of activated Stats
- RNAi:
-
RNA interference
- SHP:
-
SH2-containing phosphatases
- shRNA:
-
Short hairpin RNA
- siRNAs:
-
Small interfering RNAs
- SOCS:
-
Suppressors of cytokine signaling
- Stat3:
-
Signal transducer and activator of transcription 3
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424
Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132
Lee SS, Shin HS, Kim HJ et al. Analysis of prognostic factors and 5-year survival rate in patients with hepatocellular carcinoma: a single-center experience. Korean J Hepatol. 2012;18:48–55
Morise Z, Kawabe N, Tomishige H et al. Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20:14381–14392
Lim C, Shinkawa H, Hasegawa K et al. Salvage liver transplantation or repeat hepatectomy for recurrent hepatocellular carcinoma: an intent-to-treat analysis. Liver Transplant. 2017;23:1553–1563
Kondo Y, Kimura O, Shimosegawa T. Radiation therapy has been shown to be adaptable for various stages of hepatocellular carcinoma. World J Gastroenterol. 2015;21:94–101
Huang SX, Wu YL, Tang CW et al. Prophylactic hepatic artery infusion chemotherapy improved survival after curative resection in patients with hepatocellular carcinoma. Hepatogastroenterology. 2015;62:122–125
Ladju RB, Pascut D, Massi MN et al. Aptamer: A potential oligonucleotide nanomedicine in the diagnosis and treatment of hepatocellular carcinoma. Oncotarget. 2017;9:2951–2961
Ogunwobi OO, Harricharran T, Huaman J, Galuza A, Odumuwagun Q, Tan Y, Ma GX, Nguyen MT. Mechanisms of hepatocellular carcinoma progression. World J Gastroenterol. 2019;25:2279–2293
Girnita L, Worrall C, Takahashi SI et al. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci. 2014;71:2403–2427
Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34
LeRoith D, Werner H, Beitner-Johnson D, Roberts CT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163
Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518
Werner H. For debate: the pathophysiological significance of IGF-I receptor overexpression: new insights. Pediatr Endocrinol Rev. 2009;7:2–5
Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2014;31:805
Adachi Y, Yamamoto H, Ohashi H et al. A candidate targeting molecule of insulin-like growth factor-I receptor for gastrointestinal cancers. World J Gastroenterol. 2010;16:5779–5789
Yeo CD, Park KH, Park CK et al. Expression of insulin-like growth factor 1 receptor (IGF-1R) predicts poor responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer patients harboring activating EGFR mutations. Lung Cancer. 2015;87:311–317
Sun WY, Yun HY, Song YJ et al. Insulin-like growth factor 1 receptor expression in breast cancer tissue and mammographic density. Mol Clin Oncol. 2015;3:572–580
Nakajima N, Kozu K, Kobayashi S et al. The expression of IGF-1R in Helicobacter pylori-infected intestinal metaplasia and gastric cancer. J Clin Biochem Nutr. 2016;59:53–57
Ryan PD, Goss PE. The emerging role of the insulin-like growth factor pathway as a therapeutic target in cancer. Oncologist. 2008;13:16–24
Kamrava M, Gius D, Casagrande G, Kohn E. Will targeting insulin growth factor help us or hurt us?: An oncologist’s perspective. Ageing Res Rev. 2011;10:62–70
Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:435–444
Kim WY, Prudkin L, Feng L et al. Epidermal growth factor receptor and K-Ras mutations and resistance of lung cancer to insulin-like growth factor 1 receptor tyrosine kinase inhibitors. Cancer. 2012;118:3993–4003
Subramani R, Lopez-Valdez R, Arumugam A et al. Targeting Insulin-Like Growth Factor 1 Receptor Inhibits Pancreatic Cancer Growth and Metastasis. PLoS One. 2014;9:e97016
Bie CQ, Liu XY, Cao MR et al. Lentivirus-mediated RNAi knockdown of insulin-like growth factor-1 receptor inhibits the growth and invasion of hepatocellular carcinoma via down-regulating Midkine expression. Oncotarget. 2016;7:79305–79318
Xiong A, Yang Z, Shen Y, Zhou J, Shen Q. Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers. 2014;6:926–957
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576
Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH. Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem. 2000;275:15099–15105
Hubbard SR, Miller WT. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol. 2007;19:117–123
Belinsky MG, Rink L, Cai KQ et al. The insulin-like growth factor system as a potential therapeutic target in gastrointestinal stromal tumors. Cell Cycle. 2008;7:2949–2955
Steller MA, Delgado CH, Bartels CJ, Woodworth CD, Zou Z. Overexpression of the insulin-like growth factor-1 receptor and autocrine stimulation in human cervical cancer cells. Cancer Res. 1996;56:1761–1765
Kim SO, Park JG, Lee YI. Increased expression of the insulin-like growth factor I (IGF-I) receptor gene in hepatocellular carcinoma cell lines: implications of IGF-I receptor gene activation by hepatitis B virus X gene product. Cancer Res. 1996;56:3831–3836
Tovar V, Alsinet C, Villanueva A et al. IGF activation in a molecular subclass of hepatocellular carcinoma and pre-clinical efficacy of IGF-1R blockage. J Hepatol. 2010;52:550–559
Sanchez-Lopez E, Flashner-Abramson E, Shalapour S et al. Targeting colorectal cancer via its microenvironment by inhibiting IGF-1 Receptor-insulin receptor substrate and STAT3 signalingscript. Oncogene. 2016;35:2634–2644
Wu J, Du J, Fu X et al. Iciartin, a novel FASN inhibitor, exerts anti-melanoma activities through IGF-1R/STAT3 signaling. Oncotarget. 2016;7:51251–51269
Muramatsu T. Midkine: a promising molecule for drug developmentto treat diseases of the central nervous system. Curr Pharm Des. 2011;17:410–423
Luo J, Wang X, Xia Z et al. Transcriptional factor specificity protein 1 (SP1) promotes the proliferation of glioma cells by up-regulating midkine (MDK). Mol Biol Cell. 2015;26:430–439
Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–143
Ibusuki M, Fujimori H, Yamamoto Y et al. Midkine in plasma as a novel breast cancer marker. Cancer Sci. 2009;100:1735–1739
Muramaki M, Miyake H, Hara I, Kamidono S. Introduction of midkine gene into human bladder cancer cells enhances their malignant phenotype but increases their sensitivity to antiangiogenic therapy. Clin Cancer Res. 2003;9:5152–5160
Krzystek-Korpacka M, Diakowska D, Grabowski K, Gamian A. Tumor location determines midkine level and its association with the disease progression in colorectal cancer patients: a pilot study. Int J Colorectal Dis. 2012;27:1319–1324
Koide N, Hada H, Shinji T et al. Expression of the midkine gene in human hepatocellular carcinomas. Hepatogastroenterology. 1999;46:3189–3196
He G, Yu GY, Temkin V et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297
Calvisi DF, Ladu S, Gorden A et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–1128
Dai LC, Yao X, Lu YL et al. Expression of midkine and its relationship with HBV infection in hepatocellular carcinomas. Zhonghua yi xue za zhi. 2003;83:1691–1693
Wormald S, Hilton DJ. Inhibitors of cytokine signal transduction. J Biol Chem. 2004;79:821–824
Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387
Kile BT, Alexander WS. The suppressors of cytokine signalling (SOCS). Cell Mol Life Sci. 2001;58:1627–1635
Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. Bioessays. 1999;21:47–52
Fukushima N, Sato N, Sahin F, Su GH, Hruban RH, Goggins M. Aberrant methylation of suppressor of cytokine signalling-1 (SOCS-1) gene in pancreatic ductal neoplasms. Br J Cancer. 2001;89:338–343
Nagai H, Kim YS, Lee KT et al. Inactivation of SSI-1, a JAK/STAT inhibitor, in human hepatocellular carcinomas, as revealed by two-dimensional electrophoresis. J Hepatol. 2001;34:416–421
Yoshikawa H, Matsubara K, Qian GS et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet. 2001;28:29–35
Acknowledgments
The authors appreciate the patients, study investigators, and staff who participated in this study.
Funding
This study was supported by Grants from Shenzhen Science and Technology Project of China (No. JCYJ20160428100513152); Science and Technology Funding Project of Hunan Province, China (No. 2017SK4010); 7th batch Key Laboratory Project of Colleges and Universities of Hunan Province (2019–301); Medical Science and Technology Research Fund of Guangdong Province (No. A2019256); Natural Science Foundation of Guangdong Province (No. 2018A0303130302); and Medical Science and Technology Foundation of Guangdong Province (No. A2018011).
Author information
Authors and Affiliations
Contributions
S.H.T. designed the study. C.Q.B., Y.F.C., H.J.T., Q.L., L.Z., X.J.P., S.Y., J.Q.L., J.L.L., and S.L.W. performed the experiments. C.Q.B., Y.F.C., H.J.T., and Q.L. conducted statistical analysis. C.Q.B., Y.F.C., and S.H.T. wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics approval and consent to participate
The study was approved by the medical ethics committee of the First Affiliated Hospital of Jinan University, China. Written consent was obtained from all participants. This study conforms to the Declaration of Helsinki.
Consent for publication
Written informed consent for publication was obtained from the patients. All authors have agreed to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bie, C., Chen, Y., Tang, H. et al. Insulin-Like Growth Factor 1 Receptor Drives Hepatocellular Carcinoma Growth and Invasion by Activating Stat3-Midkine-Stat3 Loop. Dig Dis Sci 67, 569–584 (2022). https://doi.org/10.1007/s10620-021-06862-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-06862-1