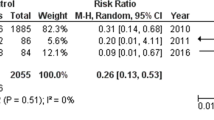
Abstract
Plecanatide, a uroguanylin analog, activates the guanylate cyclase C receptors in the epithelial lining of the gastrointestinal tract in a pH-dependent fashion initiating (1) the conversion of intracellular guanosine triphosphate to cyclic guanosine monophosphate, which increases the activity of the cystic fibrosis transmembrane conductance regulator to increase chloride and bicarbonate secretion into the intestinal lumen and (2) a decrease in activity of the sodium-hydrogen ion exchanger. The resulting ionic shifts cause an increase in lumenal fluid to facilitate digestion. Plecanatide has been approved by the FDA for use in chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation. This manuscript is a critical assessment of the therapeutic efficacy and potential risks associated with the use of plecanatide in CIC. The discussion of CIC as a clinical and investigative disorder focuses on the importance of this problem as well and the difficulties involved in clinical management and scholarly investigation of a symptom arising from multiple pathophysiologic mechanisms. Clinical data from studies of recently approved drugs for CIC are utilized to construct a platform for thoughtful understanding of CIC and of how changes in investigation guidelines influence the interpretation of study data and guide symptom management. Plecanatide is a safe and effective medication for the management of adults with CIC.

Similar content being viewed by others
References
Mearin F, Lacy BE, Chang L, et al. C2, functional constipation. In: Functional gastrointestinal disorders: disorders of gut–brain interaction. Raliegh: The Rome Foundation; 2016. p. 1002–15.
Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systemic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–91.
Herrick LM, Spalding WM, Saito YA, et al. A case-control comparison of direct healthcare-provider medical costs of chronic idiopathic constipation and irritable bowel syndrome with constipation in a community-bases cohort. J Med Econ. 2017;20:273–9.
Drossman DA, Richter JE, Talley NJ, et al. The functional gastrointestinal disorders: diagnosis, pathophysiology and treatment. McLean: Degnon Associates; 1994.
Mearin F, Lacy BE, Chang L, et al. (2016) C2, functional constipation. In: Functional gastrointestinal disorders: disorders of gut–brain interaction. Raliegh: The Rome Foundation; 2016. p. 628.
Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109:S2–26.
Kamm MA, Muller-Lissner S, Talley NJ, et al. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gasterenterol. 2005;100:362–72.
Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol. 2008;103:170–7.
Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365:527–36.
Miner PB, Koltun AD, Weiener GJ, et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017;112:613–21.
DeMicco M, Barrow L, Hickey B, et al. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Ther Adv Gastroenterol. 2017;10:837–51.
Schoenfeld P, Lacy BE, Chey WD, et al. Low-dose linaclotide (72 μg) for chronic idiopathic constipation: a 12-week, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2018;113:105–14.
Nelson AD, Camilleri M, Chirapongsathorn S, et al. Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta-analysis. Gut. 2017;66:1611–22.
Rodriguez-Stanley S, Zubaidi S, Proskin HM, et al. Effect of tegaserod on esophageal pain threshold, regurgitation and symptom relief in patients with functional heartburn and mechanical sensitivity. Clin Gasterenterol Hepatol. 2006;4:442–50.
US Food and Drug Administration. Zelnorm (tegaserod maleate) Information. https://www.fda.gov/Drugs/DrugSaftey/ucm103223.thm. Accessed 28 Nov 2018.
Omer A, Quigley EMM. An update on prucalopride in the treatment of chronic constipation. Ther Adv Gastroenterol. 2017;10:877–8.
Shin A, Camilleri M, Kolar G, et al. Sysstematic review with meta-analysis: highly selective 5-HT4 agponists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther. 2014;39:239–53.
Chey WD, Camilleri M, Chang L, et al. A randomized placebo-controlled phase IIb trail of a3309, a bile acid transport inhibitor, for chronic idiopathic constipation. Am J Gastroenterol. 2011;106:1803–12.
Miner PB Jr. Elobixibat, the first-in-class ileal bile acid transporter inhibitor, for the treatment of chronic idiopathic constipation. Expert Opin Pharmacother. 2018;19:1381–8.
Chey WD, Lembo AJ, Rosenbaum DP. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763–74.
Rutter K, Maxwell D. Disease of the alimentary system: constipation and laxative abuse. Br Med J. 1976;2:997–1000.
Dufour P, Gendre P. Long-term mucosal alterations by sennosides and related compounds. Pharmacology. 1988;36(Suppl 1):194–202.
Wald A. Is chronic use of stimulant laxatives harmful to the colon? J Clin Gastroenterol. 2003;36:386–9.
Morales MA, Hernandez Bustamante S, et al. Is senna laxative use associated to cathartic colon, genotoxicity, or carcinogenicity? J Toxicol. 2009;2009:Article ID 287247. https://doi.org/10.1155/2009/287247.
Brancale A, Shailubhai K, Ferla S, et al. Therapeutically targeting guanylate cyclase-C: computational modeling of plecanatide, a uroguanylin analog. Pharm Res Perspect. 2017;5:e00295. https://doi.org/10.1002/prp2.295.
Package Insert for TRULANCE Revised: 01/2017.
Shailubhai K, Comiskey S, Foss JA, et al. Plecanatide, an oral guanuylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig Dis Sci. 2013;58:2580–6.
Shailubhai K, Barrow L, Talluto, et al. Plecanatide, a guanylate cyclase-C agonist, improves bowel habits and symptoms associated with chronic constipation in a Phase 2a clinical study. In: Abstract P1174 presented at the American College of Gastroenterology 2011 Meeting.
Barish CF, Griffin P. Safety and tolerability of plecanatide in patients with chronic idiopathic constipation: long-tern evidence from an open-label study. Curr Med Res Opin. 2018;34:751–5.
PubChem. Compound Summary for CID 70693500 (plecanatide). https://pubchem.ncbi.nlm.nih.gov.
Shah E, Kim S, Chong K, et al. Evaluation of Harm in the pharmacotherapy of irritable bowel syndrome. Am J Med. 2012;125:381–93.
McRorie J, Zorich N, Riccardi K, et al. Effects of Olestra and sorbitol consumption on objective measures of diarrhea: Impact of stool viscosity on common gastrointestinal symptoms. Regul Toxicol Pharmacol. 2000;31:59–67.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was provided for the development of this manuscript.
Conflict of interest
Philip B. Miner Jr has no competing interests.
Informed consent
All studies conducted and referenced in this manuscript adhered to the most rigorous guidelines for informed consent and protocol development in order to obtain and maintain IRB oversight.
Rights and permissions
About this article
Cite this article
Miner, P.B. Benefit–Risk Assessment of Plecanatide in the Treatment of Chronic Idiopathic Constipation. Drug Saf 42, 603–615 (2019). https://doi.org/10.1007/s40264-018-0781-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0781-9