Abstract
Background and Aim
Simaba ferruginea A.St.-Hil. Popularly known as “calunga,” is a typical Brazilian cerrado plant whose rhizomes are popular for treating diarrhea.
Aims
The aim of this study was to evaluate the spasmolytic activity and the antidiarrheal effect of the ethanolic extract obtained from S. ferruginea (Sf-EtOH).
Methods
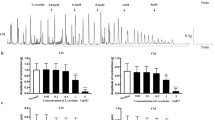
Ileal segments (1–2 cm) from male Wistar rats were mounted in isolated organ baths and connected to a force transducer, and then to an amplifier which was connected to a computer (AVS Projetos/São Paulo-SP). After stabilization for 60 min, under tension (1 gf), two submaximal contractions were induced with KCl 40 mM or carbachol 10−6 M on ileal segments. During the third tonic and sustained contraction, Sf-EtOH was added in cumulative concentrations to the organ bath. Incubations with L-NAME (10−4 M), ODQ (10−5 M), TEA+ (5 or 1 mM), glibenclamide (10−5 M), or apamine (100 nM) were prepared (n = 5), separately and used to verify the involvement of the nitric oxide synthase, guanylate cyclase, and potassium channels in the relaxing effect. The results were expressed as mean ± standard error of the mean and were statistically evaluated using one-way ANOVA followed by Bonferroni test, when necessary *p < 0.05.
Results
Sf-EtOH promotes relaxation on rat isolated ileum pre-contracted with CCh and KCl in a concentration-dependent manner. Sf-EtOH also inhibited ileum contractions against cumulative concentrations of carbachol (CCh), KCl, and CaCl2, shifting the curves to the right in a non-parallel manner with an Emax reduction. In the presence of potassium channel blockers, Sf-EtOH shifted the curves to the right with a reduction of Emax, suggesting the involvement of BKCa, KATP, and SKCa in its spasmolytic effect. In the presence of L-NAME or ODQ, the relaxation curves were shifted to the right, suggesting the involvement of this pathway in Sf-EtOH spasmolytic effect.
Conclusions
Sf-EtOH acts in a concentration-dependent manner, involving the positive modulation of K+ channels and NO pathway.










Similar content being viewed by others
References
Pokale S, Kushwaha R. A review on antidiarrhoeal activity of herbals. Int J Res Pharm Biomed Sci. 2011;2:1357–1362.
Andrade FP, Muniz RM, Wenzel SA, et al. Medicinal plants used for cancer survivors in the treatment and prevention of this disease. Rev Enferm UFPE On Line. 2011;5:944–950.
Santos RL, Guimaraes GP, Nobre MSC, et al. Analysis about phytotherapy as an integrating practice in the Brazilian Unified Health System. Rev Bras Plant Med. 2011;13:486–491.
Vanzeler MLA, Barros WM, Nasello AG, et al. Preliminary trials on female Wistar rats with hydroethanolic extract from “calunga” (Simaba ferruginea St. Hil.) in the gestational stages of implantation, organogenesis and fetal period: interference on offspring. Rev Bras Plant Med. 2015;17:454–461.
Noldin VF, Martins DTO, Marcello CM, et al. Phytochemical and antiulcerogenic properties of rhizomes from Simaba ferruginea A.St.-Hil.(Simaroubaceae). Z Naturforsch C. 2005;60:701–706.
Silva Junior I, Cechinel Filho V, Zacchino SA, et al. Antimicrobial screening of some medicinal plants from Mato Grosso Cerrado. Rev Bras Farmacogn. 2009;19:242–248.
Mellstrom B, Savignac M, Gomez-Villafuertes R, et al. Ca 2 + -operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev. 2008;88:421–449.
Ogut O, Brozovich FV. Regulation of force in vascular smooth muscle. J Mol Cell Cardiol. 2003;35:347–355.
Rembold CM. Electromechanical and pharmacomechanical coupling. In: Bárány M, ed. Biochemistry of Smooth Muscle Contraction; 1996:227–239.
Sato Y, He JX, Nagai H, et al. Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine. Biolog Pharm Bull. 2007;30:145–149.
Sun YD, Benishin CG. K+ channel openers relaxe longitudinal muscle of guinea-pig ileum. Eur J Pharmacol. 1994;271:453–459.
Silva-Filho JC, Oliveira NNPM, Arcanjo DDR, et al. Investigation of mechanisms involved in (−)-borneol-induced vasorelaxant response on rat thoracic aorta. Basic Clin Pharmacol Toxicol. 2011;110:171–177.
Ishii TM, Maylie J, Adelman JP. Determinants of apamin and d- tubocurarine block in SK potassium channels. J Biol Chem. 1997;272:23195–23200.
Knot HT, Brayden EJ, Nelson MT. Calcium channels and potassium channels. In: Bárány M, ed. Biochemistry of Smooth Muscle Contraction; 1996:203–219.
Bastos VP, Brito TS, Lima FJ, et al. Inhibitory effect of 1,8-cineole on guinea pig airway challenged with ovalbumin involves a preferential action on electromechanical coupling. Clin Exp Phamacol Phisiol. 2009;36:1120–1126.
Matsuyama H, Thapaliya S, Takewaki T. Cyclic GMP-associated apamin- sensitive nitrergic slow inhibitory junction potential in the hamster ileum. Br J Pharmacol. 1999;128:830–836.
Awouters F, Megens A, Verlinden M, et al. Loperamide: survey of studies on mechanism of its antidiarrheal activity. Dig Dis Sci. 1993;38:977–995. https://doi.org/10.1007/BF01295711.
Vasconcelos LHC, Correia ACC, Souza ILL, et al. Flavonoid galetin 3, 6-dimethyl ether attenuates guinea pig ileum contraction through K+ channel activation and decrease in cytosolic calcium concentration. Eur J Pharmacol. 2015;767:52–60.
Abdel-Latif AA. Calcium mobilizing receptors, polyphospholinositides, generation of second messengers and contraction in mammalian smooth muscle: historical perspectives and current status. Life Sci. 1989;45:757–786.
Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047–5061.
Lima JT, Almeida JRGS, Barbosa-Filho JM, et al. Spasmolytic action of diplotropin, a furanoflavan from Diplotropis ferruginea benth, involves calcium blockade in guinea-pig ileum. Z Naturforsch B. 2005;60:1093–1100.
Nouailhetas VLA, Shimuta SI, Paiva ACM, et al. Calcium and sodium dependence of the biphasic response of the guinea-pig ileum agonists. Eur J Pharmacol. 1985;116:41–47.
Honda K, Takano Y, Kamiya H. Involvement of protein kinase C in muscarinic agonist-induced contractions of guinea pig ileal longitudinal muscle. Gen Pharmacol. 1996;27:957–961.
Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC). Br J Pharmacol. 2011;164:321–324.
Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83:2015–2242.
Quest U, Cook NS. Moving together: Kþ-channel openers and ATP-sensitive Kþ-channels. Trends Pharmacol Sci. 1989;10:431–435.
Vogalis F. Potassium channels in gastrointestinal smooth muscle. J Auton Pharmacol. 2000;20:207–219.
Moradi M, Rafieian-Koupaei M, Imani-Rastabi R, et al. Antispasmodic effects of yarrow (Achillea millefolium L.) extract in the isolated ileum of rat. Afr J Tradit Complement Altern Med. 2013;10:499–503.
Archer SL. Potassium channels and erectile dysfunction. Vascul Pharmacol. 2002;38:61–71.
Baranowska MH, Kozlowska H, Korbut A, et al. Potassium channels in blood vessels: their role in health and disease. Postepy Hig Med Dosw. 2007;61:596–605.
Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:799–822.
Jackson WS. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–121.
Douglas SC, Thiago SLA, Nayara AS, et al. Sulphated polysaccharide isolated from the seaweed Gracilaria caudata exerts an antidiarrhoeal effect in rodents. Basic Clin Pharmacol Toxicol. 2016;118:440–448.
Thiago SLA, Douglas SC, Nayara AS, et al. Antidiarrheal activity of cashew GUM, a complex Heteropolysaccharide extracted from exudate of Anacardium occidentale L. in rodents. J Ethnopharmacol. 2015;174:299–307.
Hideyuki N, Noriko S, Hideaki N, et al. Possible role of potassium channels in mu-receptor-mediated inhibition and muscarinic autoinhibition in acetylcholine release from myenteric plexus of guinea pig ileum. Jpn J Pharmacol. 2000;82:343–349.
Johnson SM, Pillai NP. Hyperpolarization of myenteric neurons by opioids does not involve cyclic adenosine-3′, 5′-monophosphate. Neuroscience. 1990;36:299–304.
Michihisa M, Christie MJ, Alan NR. Single potassium channels opened by opioids in rat locus ceruleus neurons. Proc Natl Acad Sci. 1989;86:3419–3422.
Acknowledgments
We are thankful to the UFPI (Federal University of Piauı, Brazil), CNPq (Conselho Nacional de Desenvolvimento Cientıfico e Tecnológico, Brazil), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nıvel Superior, Brazil) for the scholarships and Boris Timah Acha for reviewing the English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almendra, R.B., da Fonseca, O.C., Nunes, D.B. et al. Involvement of Potassium Channels, Nitric Oxide Synthase, and Guanylate Cyclase in the Spasmolytic Effect of Simaba ferruginea A.St.-Hil on Rat Isolated Ileum. Dig Dis Sci 64, 3104–3114 (2019). https://doi.org/10.1007/s10620-019-05667-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05667-7