Abstract
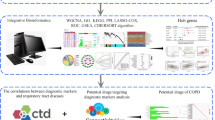
The stimulation of interleukin-1β (IL-1β) is the risk factor for temporomandibular joint osteoarthritis (TMJOA). We aim to investigate IL-1β stimulation-related gene and signal pathways in synovial fluid-derived mesenchymal stem cells (SF-MSCs) inflammatory activation to predict the occurrence of TMJOA. The microarray dataset GSE150057 was downloaded from the gene expression omnibus (GEO) database, and principal component analysis (PCA) was performed on the involved genes to obtain differential genes (DEGs). Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway were performed based on the DAVID database. The protein–protein interaction (PPI) network was constructed by the STRING database to identify hub genes. Based on the correlation between differential expression levels of lncRNAs and mRNAs, the co-expression network of lncRNA-mRNA was established. A total of 200 DEGs were obtained. Among 168 differential mRNAs, 126 were up-regulated and 42 were down-regulated; among 32 differential lncRNAs, 23 were up-regulated and 9 were down-regulated. Then, GO analysis showed that DEGs were mainly involved in signal transduction, inflammation, and apoptosis processes. KEGG pathway mainly involved the TNF signaling pathway, NF-κB signaling pathway, NOD-like receptor signaling pathway, and cytokine-cytokine-receptor interaction. Ten hub genes were recognized by PPI analysis, including CXCL8, CCL2, CXCL2, NFKBIA, CSF2, IL1A, IRF1, VCAM1, NFKB1, and TNFAIP3. In conclusion, our study has indicated the role of IL-1β stimulation in the progression of SF-MSCs inflammation and predicted DEGs and downstream pathways.







Similar content being viewed by others
Data availability
The raw data of this study are derived from the GEO data portal (https://www.ncbi.nlm.nih.gov/geo/), which is a publicly available database.
Code availability
The code could be obtained from the corresponding author upon reasonable request.
References
Afonso V, Champy R, Mitrovic D, Collin P, Lomri A (2007) Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine 74:324–329
Bonfante HL, Almeida CS, Abramo C, Grunewald STF, Levy RA, Teixeira HC (2017) CCL2, CXCL8, CXCL9 and CXCL10 serum levels increase with age but are not altered by treatment with hydroxychloroquine in patients with osteoarthritis of the knees. Int J Rheum Dis 20:1958–1964
Cristino S, Piacentini A, Manferdini C, Codeluppi K, Grassi F, Facchini A, Lisignoli G (2008) Expression of CXC chemokines and their receptors is modulated during chondrogenic differentiation of human mesenchymal stem cells grown in three-dimensional scaffold: evidence in native cartilage. Tissue Eng Part A 14:97–105
Grässel S, Zaucke F, Madry H (2021) Osteoarthritis: novel molecular mechanisms increase our understanding of the disease pathology. J Clin Med 10:1938
Henderson B, Pettipher ER (1989) Arthritogenic actions of recombinant IL-1 and tumour necrosis factor alpha in the rabbit: evidence for synergistic interactions between cytokines in vivo. Clin Exp Immunol 75:306–310
Hwang HS, Lee MH, Choi MH, Kim HA (2019) NOD2 signaling pathway is involved in fibronectin fragment-induced pro-catabolic factor expressions in human articular chondrocytes. BMB Rep 52:373–378
Israel HA, Diamond B, Saed-Nejad F, Ratcliffe A (1998) Osteoarthritis and synovitis as major pathoses of the temporomandibular joint: comparison of clinical diagnosis with arthroscopic morphology. J Oral Maxillofac Surg 56:1023–1027
Jorgenson KD, Hart DA, Krawetz R, Sen A (2018) Production of adult human synovial fluid-derived mesenchymal stem cells in stirred-suspension culture. Stem Cells Int 2018:8431053
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H (2011) Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 7:33–42
Kellesarian SV, Al-Kheraif AA, Vohra F, Ghanem A, Malmstrom H, Romanos GE, Javed F (2016) Cytokine profile in the synovial fluid of patients with temporomandibular joint disorders: a systematic review. Cytokine 77:98–106
Kim YK, Kim SG, Kim BS, Lee JY, Yun PY, Bae JH, Oh JS, Ahn JM, Kim JS, Lee SY (2012) Analysis of the cytokine profiles of the synovial fluid in a normal temporomandibular joint: preliminary study. J Craniomaxillofac Surg 40:e337-341
Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR (2005) Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 52:128–135
Lei J, Fu Y, Zhuang Y, Zhang K, Lu D (2019) LncRNA SNHG1 alleviates IL-1β-induced osteoarthritis by inhibiting miR-16-5p-mediated p38 MAPK and NF-κB signaling pathways. Biosci Rep. https://doi.org/10.1042/BSR20191523
Li J, Huang Y, Song J, Li X, Zhang X, Zhou Z, Chen D, Ma PX, Peng W, Wang W, Zhou G (2018) Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomater 79:202–215
Liu Y, Mi B, Lv H, Liu J, Xiong Y, Hu L, Xue H, Panayi AC, Liu G, Zhou W (2018) Shared KEGG pathways of icariin-targeted genes and osteoarthritis. J Cell Biochem 120:7741–7750
Liu C, Ren S, Zhao S, Wang Y (2019) LncRNA MALAT1/MiR-145 adjusts IL-1β-induced chondrocytes viability and cartilage matrix degradation by regulating ADAMTS5 in human osteoarthritis. Yonsei Med J 60:1081–1092
Longobardi L, Jordan JM, Shi XA, Renner JB, Schwartz TA, Nelson AE, Barrow DA, Kraus VB, Spagnoli A (2018) Associations between the chemokine biomarker CCL2 and knee osteoarthritis outcomes: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage 26:1257–1261
Massicotte F, Lajeunesse D, Benderdour M, Pelletier JP, Hilal G, Duval N, Martel-Pelletier J (2002) Can altered production of interleukin-1beta, interleukin-6, transforming growth factor-beta and prostaglandin E(2) by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis Cartilage 10:491–500
Mobasheri A (2013) The future of osteoarthritis therapeutics: emerging biological therapy. Curr Rheumatol Rep 15:385
Page Thomas DP, King B, Stephens T, Dingle JT (1991) In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann Rheum Dis 50:75–80
Raghu H, Lepus CM, Wang Q, Wong HH, Lingampalli N, Oliviero F, Punzi L, Giori NJ, Goodman SB, Chu CR, Sokolove JB, Robinson WH (2017) CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis 76:914–922
Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, Lindstrom TM, Hwang I, Boyer KA, Andriacchi TP, Robinson WH (2012) Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther 14:R7
Wang T, He C (2018) Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev 44:38–50
Wang XD, Zhang JN, Gan YH, Zhou YH (2015) Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res 94:666–673
Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D (2014) The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014:561459
Acknowledgements
All authors would like to thank the GEO database, which made the data available.
Funding
This study was financed in part by the National Natural Science Foundation of China, Grant number: 81873720, the Medical Science and Technology Planning Project of Ningbo City, Zhejiang Province, Grant number: 2020Y05, and the Open project of Zhejiang Provincial Key Laboratory of Dental Biomedicine Research, Grant number: 2021M010, 2021M004.
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation of this manuscript. YL, RT, and XW: drafted the manuscript. YL and SS: performed data analysis. BY and YY: conceived the study. YY and BY: reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare there are no competing interests.
Ethical approval
Not necessary.
Consent for publications
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lou, Y., Tao, R., Weng, X. et al. Bioinformatics analysis of synovial fluid-derived mesenchymal stem cells in the temporomandibular joint stimulated with IL-1β. Cytotechnology 75, 325–334 (2023). https://doi.org/10.1007/s10616-023-00579-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-023-00579-x