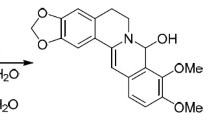
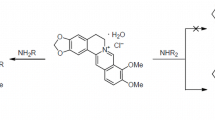
The possibility of obtaining electroneutral 8-amido derivatives of dihydroberberines was shown experimentally and with the support of quantum-chemical calculations based on the density functional theory (B3LYP/6-311+G(d,p)). In alkaline media, amides deprotonated to form amide anions, which, in turn, could add to the berberine framework at the C-8 position. Competing processes in this case were the reactions of the formation of 8-hydroxy- or 8-alkoxyberberines.
Similar content being viewed by others
References
Sun, Y.; Xun, K.; Wang, Y.; Chen, X. Anti-Cancer Drugs 2009, 20, 757.
Kulkarni, S. K.; Dhir, A. Phytother. Res. 2010, 24, 317.
Tan, W.; Li, Y.; Chen, M.; Wang, Y. Int. J. Nanomed. 2011, 1773.
McCubrey, J. A.; Abrams, S. L.; Lertpiriyapong, K.; Cocco, L.; Ratti, S.; Martelli, A. M.; Candido, S.; Libra, M.; Murata, R. M.; Rosalen, P. L.; Lombardi, P.; Montalto, G.; Cervello, M.; Gizak, A.; Rakus, D.; Steelman, L. S. Adv. Biol. Regul. 2018, 67, 190.
Li, Y.-H.; Fu, H.-G.; Su, F.; Gao, L.-M.; Tang, S.; Bi, C.-W.; Li, Y.-H.; Wang, Y.-X.; Song, D.-Q. Chem. Cent. J. 2013, 7, 117.
Vuddanda, P. R.; Chakraborty, S.; Singh, S. Expert Opin. Invest. Drugs 2010, 19, 1297.
Nechepurenko, I. V.; Salakhutdinov, N. F.; Tolstikov, G. A. Khimiya v interesakh ustoichivogo razvitiya 2010, 18, 1.
Yin, J.; Zhang, H.; Ye, J. Endocr.‚ Metab. Immune Disord.: Drug Targets 2008, 8, 99.
Hui, H.; Tang, G.; Go, V. L. W. Chin. Med. 2009, 4, 11.
Chen, Ya.; Wang, Ya.; Zhang, J.; Sun, C.; Lopez, A. Int. Scholarly Res. Not. 2011, 519371.
Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Yang, D.; Hua, L.; Ye, J.; Weng, J. PLoS One 2011, 6, e16556
Nechepurenko, I. V.; Boyarskikh, U. A.; Khvostov, M. V.; Baev, D. S.; Komarova, N. I.; Filipenko, M. L.; Salakhutdinov, N. F. Chem. Nat. Compd. 2015, 51, 916.
Liu, Y.-X.; Xiao, C.-L.; Wang, Y.-X.; Li, Y.-H.; Yang, Y.-H.; Li, Y.-B.; Bi, Ch.-W.; Gao, L.-M.; Jiang, J.-D, Song, D.-Q. Eur. J. Med. Chem. 2012, 52, 151.
Iwasa, K.; Moriyasu, M.; Yamori, T.; Turuo, T.; Lee, D.-U.; Wiegrebe, W. J. Nat. Prod. 2001, 64, 896.
Hayashi, K.; Minoda, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Bioorg. Med. Chem. Lett. 2007, 17, 1562.
Burov, O. N.; Kletskii, M. E.; Fedik, N. S.; Kurbatov, S. V.; Lisovin, A. V. Chem. Heterocycl. Compd. 2015, 51, 997.
Grycová, L.; Dostál, J.; Marek, R. Phytochemistry 2007, 68, 150.
Franceschin, M.; Rossetti, L.; D'Ambrosio, A.; Schirripa, S.; Bianco, A.; Ortaggi, G.; Savino M.; Schultes C.; Neidle, S. Bioorg. Med. Chem. Lett. 2006, 16, 1707
Bremner, J. B.; Samosorn, S. Aust. J. Chem. 2003, 56(9), 871.
Burov, O. N.; Kurbatov, S. V.; Kletskii, M. E.; Zagrebaev, A. D.; Mikhailov, I. E. Chem. Heterocycl. Compd. 2017, 53, 335.
Demekhin, O. D.; Zagrebaev, A. D.; Burov, O. N.; Kletskii, M. E.; Pavlovich, N. V.; Bereznyak, E. A.; Tsimbalistova, M. V.; Kurbatov, S. V. Chem. Heterocycl. Compd. 2019, 55, 1128.
Zagrebaev, A. D.; Burov, O. N.; Kletskii, M. E.; Lisovin, S. V.; Kurbatov, S. V.; Demekhin, O. D. Chem. Heterocycl. Compd. 2022, 58, 45.
Burov, O. N.; Kurbatov, S. V.; Morozov, P. G.; Kletskii, M. E.; Tatarov, A. V. Chem. Heterocycl. Compd. 2015, 51, 772.
Šimánek, V.; Preininger, V.; Hegerová, S.; Šantavý, F. Coll. Czech. Chem. Commun. 1972, 37, 2746.
Olleik, H.; Yacoub, T.; Hoffer, L.; Gnansounou, S. M.; Benhaiem-Henry, K.; Nicoletti, C.; Mekhalfi, M.; Pique, V.; Perrier, J.; Hijazi, A.; Baydoun, E.; Raymond, J.; Piccerelle, P.; Robin, M. Antibiotics 2020, 9, 381.
Iwasa, K.; Kamigauchi, M.; Ueki, M.; Taniguchi, M. Eur. J. Med. Chem. 1996, 31, 469.
Li, X.; Zhang, H.-J.; Li, Z.-H.; Wu, L.-Q.; Deng, A.-J.; Qin, H.-L. J. Asian Nat. Prod. Res. 2022, 24, 388.
Möhrle, H.; Biegholdt, M. Arch. Pharm. 1982, 315, 919.
Man, S.; Dostal, J.; Nečas, M.; Zák, Z.; Potaček, M. Heterocycl. Commun. 2001, 7, 243.
Grycová, L.; Hulová, D.; Maier, L.; Standara, S.; Nečas, M.; Lemière, F.; Kares, R.; Dostál, J.; Marek, R. Magn. Reson. Chem. 2008, 46, 1127.
Parr, R. G.; von Szentpály, L.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922.
Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
Becke, A. D. Phys. Rev. A 1988, 38, 3098.
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785.
Kletskii, M. Е.; Burov, О. N.; Dalinger, I. L.; Shevelev, S. А. Comput. Theor. Chem. 2014, 1033, 31.
Burov, O. N.; Kletskii, M. E.; Gulevskaya, A. V. Russ. Chem. Bull. 2013, 62, 1156.
Suzdalev, K. F.; Den'kina, S. V.; Starikova, A. A.; Dvurechensky, V. V.; Kletsky, M. E.; Burov, O. N. Mendeleev Commun. 2011, 21, 231.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A., Jr.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, J. D.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03; Gaussian, Inc.: Wallingford, 2003.
Schlegel, H. B. Theor. Chim. Acta 1984, 66, 333.
Hirsh, M.; Quapp, W. Chem. Phys. Lett. 2004, 395, 150.
Simkin, B. Ya.; Sheikhet, I. I. Quantum Chemical and Statistical Theory of Solutions: A Computational Approach; Ellis Horwood: London, 1995.
Cancès, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032.
Parr, R. G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, 1989.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(6/7), 363–367
Supplementary Information
ESM 1
(PDF 1876 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zagrebaev, A.D., Burov, O.N., Kletskii, M.E. et al. A Reaction of Berberine with Amides in Alkaline Media: An Experimental and Quantum-Chemical Study. Chem Heterocycl Comp 58, 363–367 (2022). https://doi.org/10.1007/s10593-022-03099-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03099-2