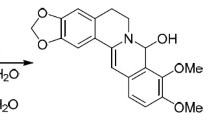
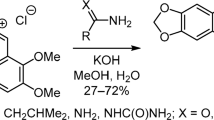
Principal differences in the interaction mechanisms of alkaloid berberine with primary and secondary amines were investigated experimentally and by quantum-chemical calculations according to density functional theory (DFT/B3LYP) with 6-31G** basis set. The nucleophilic substitution of 9-metoxy group with primary amine was shown to proceed through a stage of σ-complex formation and led to 9-alkylamino derivatives of berberine. Analogous substitution with a secondary amine did not occur due to unfavorable thermodynamic parameters. The secondary amine participated in this reaction not as the attacking nucleophile, but rather as a bifunctional catalyst of berberine hydrolysis to berberrubine. The driving force for all these processes was the stabilization of products by hydrogen bonding. Based on the obtained results, we developed a new effective method for the preparation of berberrubine, one of the key intermediates in synthetic transformations of berberine. New 9-monoalkylamino derivatives of berberine containing indole moieties were synthesized.









Similar content being viewed by others
Notes
ρ(r c ) – electron density; ∇2ρ(r c ) – the Laplacian of electron density.
References
Saha, S. K.; Khuda-Bukhsh, A. R. Eur. J. Pharmacol. 2013, 714, 239.
Siow, Y. L; Sarna, L; Karmin, O. Food Res. Int. 2011, 44, 2409.
Khazir, J.; Mir, B. A.; Pilcher, D. L.; Riley, D. L. Phytochem. Lett. 2004, 7, 173.
Crycova, L.; Dostal, J.; Marek, R. Phytochem. 2007, 68, 150.
Ji, H.-F.; Shen, L. Molecules 2011, 16, 6732.
Krivogorsky, B.; Pernat, J. A.; Douglas, K. A.; Czerniecki, N. J.; Grundt, P. Bioorg. Med. Chem. Lett. 2012, 22, 2980.
Nechepurenko, I. V.; Salakhutdinov, N. F.; Tolstikov, G. A. Khimiya v Interesakh Ustoichovogo Razvitiya 2010, 18, 1.
Shan, W.-J.; Huang, L.; Zhou, Q.; Meng, F.-Ch.; Li, X.-Sh. Eur. J. Med. Chem. 2011, 46, 5885.
Huang, L.; Shi, A.; He, F.; Li, X. Bioorgan. Med. Chem. 2010, 18, 1244.
Ma, Y.; Ou, T.-M.; Hou, J.-Q.; Lu, Y.-J.; Tan, J.-H.; Gu, L.-Q.; Huang, Zh.-Sh. Bioorg. Med. Chem. 2008, 16, 7582.
Ma, Y.; Ou, T.-M.; Tan, J.-H.; Hou, J.-Q.; Huang, Sh.-L.; Gu, L.-Q.; Huang, Zh.-Sh. Eur. J. Med. Chem. 2011, 46, 1906.
Huang, L.; Luo, Z.; He, F.; Shi, A. Bioorg. Med Chem. Let. 2010, 20, 6649.
Ma, Y.; Ou, T.-M.; Tan, J.-H.; Hou, J.-Q.; Huang, Sh.-L. Bioorg. Med. Chem. Let. 2009, 19, 3414.
Bodiwala, H. S.; Sabde, S.; Mitra, D.; Bhutani, K. K.; Singh, I. P. Eur. J. Med. Chem. 2011, 46, 1045.
Burov, O. N.; Kurbatov, S. V.; Morozov, P. G.; Kletskii, M. E.; Tatarov, A. V. Chem. Heterocycl. Compd. 2015, 51, 772. [Khim. Geterotsikl. Soedin. 2015, 51, 772.]
Lam, Y. Int. J. Pharma Sci. Res. 2014, 5, 350.
Bader, R. F. W. Atoms in Molecules. Quantum Theory [Russian translation]; Mir, Moscow, 2001.
Kariuki, B. M.; Jones, W. Acta Cryst. Sect. C 1995, 51, 1234.
Iwasa, K.; Kamigauchi, M.; Uek, M.; Taniguch, M. Eur. J. Med. Chem. 1996, 31, 469.
Gorb, L.; Podolyan, E.; Dziekonski, P.; Sokalski, W. A.; Leszczynski, J. J. Am. Chem. Soc. 2004, 126, 10119.
Jovené, C.; Jacquet, M.; Marrot, J.; Bourdreux, F.; Kletsky, M. E.; Burov, O. N.; Gonçalves, A.-M.; Goumont, F. Eur. J. Org. Chem. 2014, 6451.
Elliott, I. W. J. Heterocycl. Chem. 1967, 4, 639.
Becke, A. D. Phys. Rev. A. 1988, 38, 3098.
Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B. 1988, 37, 785.
Burov, O. N.; Kletskii, M. E.; Gulevskaya, A. V. Russ. Chem. Bull. 2013, 62, 1156. [Izv. Akad. Nauk, Ser. Khim. 2013, 1155.]
Kletskii, M. Е.; Burov, О. N.; Dalinger, I. L.; Shevelev, S. А. Comp. Theor. Chem. 2014, 1033, 31.
Suzdalev, K. F.; Den'kina, S. V.; Starikova, A. A.; Dvurechensky, V. V.; Kletsky, M. E.; Burov, O. N. Mendeleev Commun. 2011, 21, 231.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A.; Vreven, Jr. T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E., Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, J. D.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03; Gaussian, Inc.: Wallingford, 2004.
Schlegel, H. B. Theor. Chim. Acta 1984, 66, 333.
Hirsh, M.; Quapp, W. Chem. Phys. Lett. 2004, 395, 150.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Allen, F. H. Acta Crystallogr., Sect. B: Struct. Sci. 2002, B58, 380.
This work was performed within the framework of Project part of the State Assignment No.4.129.2014/K from the Ministry of Education and Science of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Supplementary information file containing the geometrical characteristics of structures 5–24 and the transition states, as well as the full and relative energy (in tabular form) for all stationary points on the reaction MEP is available online at http://link.springer.com/journal/10593.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(11/12), 997–1007
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2166 kb)
Rights and permissions
About this article
Cite this article
Burov, O.N., Kletskii, M.E., Fedik, N.S. et al. Experimental and quantum-chemical study of nucleophilic substitution mechanism in berberine. Chem Heterocycl Comp 51, 997–1007 (2015). https://doi.org/10.1007/s10593-016-1810-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1810-1