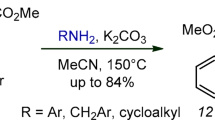
Graebe–Ullmann reaction was used to synthesize 3-aryl(methyl)-1-methyl(cyclopropyl)-4-nitro-5Н-pyrido[4,3-b]indoles containing substituents in the pyridine ring. A new scheme was developed for the synthesis of the starting 1-[6-aryl(methyl)-2-methyl(cyclopropyl)- 3-nitropyridin-4-yl]-1Н-1,2,3-benzotriazoles: 1) ammonolysis of 3-nitroisonicotinic acid ethyl esters, 2) a modified Hofmann reaction of 3-nitroisonicotinamides, 3) cleavage of ethyl (2-aryl-3-nitropyridin-4-yl)carbamates, 4) synthesis of 4-chloro-3-nitropyridines by diazotation reaction of 4-amino-3-nitropyridines, followed by nucleophilic substitution of diazo group in 4-pyridyldiazonium salts with a chlorine atom.
Similar content being viewed by others
References
(а) Аlekseyev, R. S.; Kurkin, A. V.; Yurovskaya, M. A. Chem. Heterocycl. Compd.2009, 45, 889. [Khim. Geterotsikl. Soedin.2009, 1123.] (b) Dai, J.; Dan, W.; Zhang, Y.; Wang, J. Eur. J. Med. Chem.2018, 157, 447. (c) Аlekseyev, R. S.; Kurkin, A. V.; Yurovskaya, M. A. Chem. Heterocycl. Compd.2010, 46, 777. [Khim. Geterotsikl. Soedin.2010, 963.] (d) Dong, H.; Latka, R. T.; Driver, T. G. Org. Lett.2011, 13, 2726. (e) Nissen, F.; Richard, V.; Alayrac, C.; Witulski, B. Chem. Commun. 2011, 47, 6656.
(a) Nantka-Namirski, P. Acta Pol. Pharm. 1961, 18, 449. (b) Nantka-Namirski, P. Acta Pol. Pharm. 1961, 18, 391.
Sagitullina, G. P.; Garkushenko, A. K.; Dushek, M. A.; Poendaev, N. V.; Sagitullin, R. S. Chem. Heterocycl. Compd.2011, 46, 1250. [Khim. Geterotsikl. Soedin.2010, 1546.]
Kalinowski, J.; Rykowski, A.; Nantka-Namirski, P. Pol. J. Chem.1984, 58, 125.
Yurovskaya, M. A.; Аlekseyev, R. S. Chem. Heterocycl. Compd.2014, 49, 1400. [Khim. Geterotsikl. Soedin.2013, 1507.]
(a) Marvel, C. S.; Dreger, E. E. Org. Synth.1926, 6, 40. (b) Cannon, G. W.; Whidden, H. L. J. Org. Chem.1952, 17, 685.
Shuvalov, V. Yu.; Rupp, A. S.; Fisyuk, A. S.; Kuratova, A. K.; Nefedov, A. A.; Sagitullina, G. P. ChemistrySelect2019, 4, 1696.
(a) Hossain, M. D., Kitamura, T. Bull. Chem. Soc. Jpn. 2007, 80, 2213. (b) Loudon, G. M.; Radhakrishna, A. S.; Almond, M. R.; Blodgett, J. K.; Boutin, R. H. J. Org. Chem. 1984, 49, 4272.
Uhlig, F. Angew. Chem. 1954, 66, 435.
This work received financial support from the Russian Foundation for Basic Research (grant 16-43-550144r_a) and from the Ministry of Education of the Omsk region.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(9), 844–850
Rights and permissions
About this article
Cite this article
Shuvalov, V.Y., Shestakov, A.N., Kulakova, L.A. et al. Synthesis of 4-nitro-γ-carbolines by Graebe–Ullmann reaction. Chem Heterocycl Comp 55, 844–850 (2019). https://doi.org/10.1007/s10593-019-02547-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02547-w
Keywords
- 4-amino-3-nitropyridines
- 1-[6-aryl(methyl)-2-methyl(cyclopropyl)-3-nitropyridin-4-yl]-1Н-1,2,3-benzotriazoles
- 3-aryl(methyl)- 1-methyl(cyclopropyl)-4-nitro-5Н-pyrido[4,3-b]indoles
- 4-chloro-3-nitropyridines
- ethyl (4-pyridyl)carbamates
- ethyl esters and amides of 3-nitroisonicotinic acid
- Graebe–Ullmann reaction