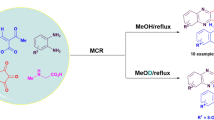
We provide a general and systematic review of all currently published data on the use of stabilized azomethine ylides derived from 11H-indeno[1,2-b]quinoxalin-11-one and 6H-indeno[1,2-b]pyrido[3,2-e]pyrazin-6-one in the synthesis of spiropyrrolidines and spiropyrrolizidines. The reaction conditions, as well as the regio- and stereoselectivity of the [3+2] cycloaddition process are discussed. Data on the biological activity of the obtained products are presented. There are 62 literature references.



Similar content being viewed by others
References
Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Padwa, A.; Pearson, W. H., Eds.; Wiley: New York, 2002, Vol. 59, p 169.
Tsuge, O.; Kanemasa, S. Adv. Heterocycl. Chem. 1989, 45, 231.
Arumugam, N.; Suresh Kumar, R.; Almansour, A. I.; Perumal, S. Curr. Org. Chem. 2013, 17, 1929.
Singh, M. S.; Chowdhury, S.; Koley, S. Tetrahedron 2016, 72, 1603.
Döndas, H. A.; Retamosa, M. G.; Sansano, J. M. Synthesis 2017, 49, 2819.
Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484.
Nájera, C.; Sansano, J. M. J. Organomet. Chem. 2014, 771, 78.
Adrio, J.; Carretero, J. C. Chem. Commun. 2014, 50, 12434.
Nájera, C.; Sansano, J. M. Curr. Top. Med. Chem. 2014, 14, 1271.
Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366.
Tseng, C.-H.; Chen, Y.-R.; Tzeng, C.-C.; Liu, W.; Chou, C.-K.; Chiu, C.-C.; Chen, Y.-L. Eur. J. Med. Chem. 2016, 108, 258.
Zhang, C.; Li, S.; Ji, L.; Liu, S.; Li, Z.; Li, S.; Meng, X. Bioorg. Med. Chem. Lett. 2015, 25, 4693.
Schepetkin, I. A.; Kirpotina, L. N.; Khlebnikov, A. I.; Hanks, T. S.; Kochetkova, I.; Pascual, D. W.; Jutila, M. A.; Quinn, M. T. Mol. Pharmacol. 2012, 81, 832.
Khan, M. S.; Munawar, M. A.; Ashraf, M.; Alam, U.; Ata, A.; Asiri, A. M.; Kousar, S.; Khan, M. A. Bioorg. Med. Chem. 2014, 22, 1195.
Zhang, C.; Li, S.; Ji, L.; Liu, S.; Li, Z.; Li, S.; Meng, X. Bioorg. Med. Chem. Lett. 2015, 25, 4693.
Ruhemann, S. J. Chem. Soc. 1910, 97, 1438.
Israel, M.; Jones, L. C.; Modest, E. J. J. Heterocycl. Chem. 1972, 9, 255.
Azizian, J.; Karimi, A. R.; Dastkhan, R.; Mohammadi, A. A.; Mohammadizadeh, M. R. J. Chem. Res. 2004, 347.
Barkov, A. Y.; Zimnitskiy, N. S.; Korotaev, V. Y.; Kutyashev, I. B.; Moshkin, V. S.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 451. [Khim. Geterotsikl. Soedin. 2017, 53, 451.]
Hamzehloueian, M.; Sarrafi, Y.; Aghaei, Z. RSC Adv. 2015, 5, 76368.
Shahrestani, N.; Salahi, F.; Tavakoli, N.; Jadidi, K.; Hamzehloueian, M.; Notash, B. Tetrahedron: Asymmetry 2015, 26, 1117.
Filatov, A. S.; Knyazev, N. A.; Ryazantsev, M. N.; Suslonov, V. V.; Larina, A. G.; Molchanov, A. P.; Kostikov. R. R.; Boitsov, V. M.; Stepakov, A. V. Org. Chem. Front. 2018, 5, 595.
Sobhi, C.; Nacereddine, A. K.; Djerourou, A.; Ríos-Gutiérrez, M; Domingo, L. R. J. Phys. Org. Chem. 2017, 30, 3637.
Kuznetsov, M. L. Russ. Chem. Rev. 2006, 75, 935. [Usp. Khim. 2006, 75, 1045.]
Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765.
Shevelev, S. A.; Starosotnikov, A. M. Chem. Heterocycl. Compd. 2013, 49, 92. [Khim. Geterotsikl. Soedin. 2013, 102.]
The Cambridge Crystallographic Data Centre (CCDC) https://www.ccdc.cam.ac.uk
Mani, K. S.; Kaminsky W.; Rajendran, S. P. New J. Chem. 2018, 42, 301.
Barkov, A. Y.; Zimnitskiy, N. S.; Kutyashev, I. B.; Korotaev, V. Y.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 1315. [Khim. Geterotsikl. Soedin. 2017, 53, 1315.]
Moemeni, M.; Arvinnezhad, A.; Samadi, S.; Tajbakhsh, M.; Jadidi, K.; Khavasi, H. R. J. Heterocycl. Chem. 2012, 49, 190.
Velikorodov, A. V.; Stepkina, N. N. Russ. J. Org. Chem. 2016, 52, 1788. [Zh. Org. Khim. 2016, 52, 1797.]
Mohammadizadeh, M. R.; Firoozi, N. Bull. Korean Chem. Soc. 2009, 30, 1877.
Nishtala, V. B.; Nanuboli, J. B.; Basavoju, S. Res. Chem. Intermed. 2017, 43, 1365.
Kathivaran, S.; Raghunathan, R. J. Heterocycl. Chem. 2014, 51, 906.
Babu, A. R. S.; Gavaskar, D.; Raghunathan, R. Tetrahedron Lett. 2012, 53, 6676.
Babu, A. R. S.; Gavaskar, D.; Raghunathan, R. J. Organomet. Chem. 2013, 745-746, 409.
Gavaskar, D.; Babu, A. R. S.; Raghunathan, R.; Dharani, M.; Balasubramanian, S. J. Organomet. Chem. 2014, 768, 128.
Gavaskar, D.; Babu, A. R. S.; Raghunathan, R.; Dharani, M.; Balasubramanian, S. Steroids 2016, 109, 1.
Liu, F.-H.; Song, Y.-B.; Zhai, L.-J.; Li, M. J. Heterocycl. Chem. 2015, 52, 322.
Babu, A. R. S.; Raghunathan, R. Synth. Commun. 2009, 39, 347.
Babu, A. R. S.; Raghunathan, R. Synth. Commun. 2008, 38, 1433.
Babu, A. R. S.; Raghunathan, R. Tetrahedron Lett. 2006, 47, 9221.
Gayathri, D.; Aravindan, P. G.; Velmurugan, D.; Ravikumar, K.; Babu, A. R. S. Acta Crystallogr., Sect. E: Crystallogr. Commun. 2005, 61, 3124.
Malathi, K.; Kanchithalaivan, S.; Kumar, R. R.; Almansour, A. I.; Kumar, R. S.; Arumugam, N. Tetrahedron Lett. 2015, 56, 6132.
Rajesh, S. M.; Bala, B. D.; Perumal, S. Tetrahedron Lett. 2012, 53, 5367.
Rani, M. A.; Kumar, S. V.; Malathi, K.; Muthu, M.; Almansour, A. I.; Kumar, R. S.; Kumar, R. R. ACS Comb. Sci. 2017, 19, 308.
Zimnitskiy, N. S.; Korotaev, V. Yu.; Barkov, A. Yu.; Kutyashev, I. B.; Sosnovskikh, V. Ya. In: From the synthesis of polyethylene to stereodivergence: the developments in chemistry over 100 years [in Russian]. Proceedings of international scientific conference; Perm, 2018, p. 120.
Karsalary, A. A.; Mohammadizadeh, M. R.; Hasaninejad, A. R.; Mohammadi, A. A.; Karimi, A. R. J. Iran. Chem. Soc. 2010, 7, 45.
Azizian, J.; Karimi, A. R.; Mohammadi, A. A.; Mohammadizadeh, M. R. Synthesis 2004, 2263.
Kathiravan, S.; Raghunathan, R.; Suresh, G.; Siva, G. V. Med. Chem. Res. 2012, 21, 3170.
Ramesh, E.; Kathiresan, M.; Raghunathan, R. Tetrahedron Lett. 2007, 48, 1835.
Pattanaik, P.; Nayak, S.; Mishra, D. R.; Panda, P.; Raiguru, B. P.; Mishra, N. P.; Mohapatra, S.; Mallampudi, N. A.; Purohit, C. S. Tetrahedron Lett. 2018, 59, 2688.
Li, M.; Gong, F.-M.; Wen, L.-R.; Li, Z.-R. Eur. J. Org. Chem. 2011, 3482.
Velikorodov, A. V.; Stepkina, N. N.; Shustova, E. A.; Ionova, V. A. Russ. J. Org. Chem. 2015, 51, 674. [Zh. Org. Khim. 2015, 51, 693.]
Barkov, A. Y.; Zimnitskiy, N. S.; Kutyashev, I. B.; Korotaev, V. Y.; Sosnovskikh, V. Yа. Chem. Heterocycl. Compd. 2018, 54, 43. [Khim. Geterotsikl. Soedin. 2017, 53, 43.]
Akondi, A. M.; Mekala, S.; Kantam, M. L.; Trivedi, R.; Chowhan, L. R.; Das, A. New J. Chem. 2017, 41, 873.
Reddy, M. S.; Chowhan, L. R.; Kumar, N. S.; Ramesh, P.; Mukkamala, S. B. Tetrahedron Lett. 2018, 59, 1366.
Rao, J. N. S.; Raghunathan, R. Tetrahedron Lett. 2015, 56, 2276.
Barkov, A. Y.; Zimnitskiy, N. S.; Korotaev, V. Y.; Kutyashev, I. B.; Moshkin, V. S.; Sosnovskikh, V. Ya. J. Fluorine Chem. 2017, 204, 37.
Barkov, A. Y.; Zimnitskiy, N. S.; Korotaev, V. Y.; Kutyashev, I. B.; Moshkin, V. S.; Sosnovskikh, V. Ya. Tetrahedron 2016, 72, 6825.
Lakshmi, N. V.; Thirumurugan, P.; Jayakumar, C.; Perumal, P. T. Synlett 2010, 955.
Rao, J. N. S.; Raghunathan, R. Tetrahedron Lett. 2015, 56, 1539.
This work was performed with financial support from the Ministry of Education and Science of the Russian Federation (contract 02.A03.21.0006) and the Russian Foundation for Basic Research (project No. 18-33-00635).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(10), 905–922
Rights and permissions
About this article
Cite this article
Korotaev, V.Y., Zimnitskiy, N.S., Barkov, A.Y. et al. Stabilized azomethine ylides derived from indeno[1,2-b]quinoxalinones in [3+2] cycloaddition reactions with electrophilic alkenes. Chem Heterocycl Comp 54, 905–922 (2018). https://doi.org/10.1007/s10593-018-2369-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2369-9