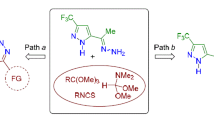
New podands containing pyrrole or pyrazoline fragments have been synthesized. A hypothesis was proposed that closure of the pyrrole ring in the presence of alumina nanoparticles may proceed through 1,4-addition of nitroethane to the chalcone podand to give the Michael adduct rather than through an iminium intermediate usually observed in the reactions of α,β-unsaturated ketones (chalcones) in the presence of Lewis acids. Moderate tuberculostatic activity was found for the starting chalcone podands. Much greater such activity was found upon subsequent modification of the chalcone fragment to give a five-membered heterocycle. Pyrrole derivatives displayed greatest activity among the hetaryl podands obtained.





Similar content being viewed by others
References
K.-S. Jeong, Y. L. Cho, and S. Y. Pyun, Tetrahedron Lett., 36, 2827 (1995).
O. V. Fedorova, G. L. Rusinov, G. G. Mordovskoi, M. N. Zueva, M. A. Kravchenko, I. G. Ovchinnikova, and O. N. Chupakhin, Khim.-farm. Zh., 31, No. 7, 21 (1997).
V. A. Potemkin, M. A. Grishina, O. V. Fedorova, G. L. Rusinov, I. G. Ovchinnikova, and R. I. Ishmetova, Khim.-farm. Zh., 37, No. 9, 17 (2003). [Pharm. Chem. J., 37, 468 (2003)].
M. A. Ali and M. Shaharyar, Bioorg. Med. Chem., 15, 1896 (2007).
M. Shaharyar, A. A. Siddiqui, M. A. Ali, D. Sriram, and P. Yogeewsari, Bioorg. Med. Chem. Lett., 16, 4571 (2006).
I. G. Ovchinnikova, O. V. Fedorova, P. A. Slepukhin, I. A. Litvinov, and G. L. Rusinov, Kristallografiya, 54, 37 (2009). [Crystallogr. Repts., 54, 31 (2009)].
H. Shiraishi, T. Nishitani, S. Sakaguchi, and Y. Ishii, J. Org. Chem., 63, 6234 (1998).
B. C. Ranu and A. Hajra, Tetrahedron, 57, 4767 (2001).
E. J. Roskamp, P. S. Dragovich, J. B. Hartung, Jr., and S. F. Pedersen, J. Org. Chem., 54, 4736 (1989).
O. V. Fedorova, M. S. Valova, Yu. A. Titova, I. G. Ovchinnikova, A. N. Grishakov, M. A. Uimin, A. A. Mysik, A. E. Ermakov, G. L. Rusinov, and V. N. Charushin, Kinetika i Kataliz, 52, 234 (2011). [Kinet. Catal., 52, 226 (2011)].
Yu. A. Titova, O. V. Fedorova, I. G. Ovchinnikova, G. L. Rusinov, and V. N. Charushin, Zh. Prikl. Khim., 85, 641 (2012). [Russ. J. App. Chem., 85, 656 (2012)].
O. V. Fedorova, O. V. Koryakova, M. S. Valova, I. G. Ovchinnikova, Yu. A. Titova, G. L. Rusinov, and V. N. Charushin, Kinetika i Kataliz, 51, 590 (2010). [Kinet. Catal., 51, 590 (2010)].
A. A. Davydov, IR Spectroscopy in the Chemistry of Oxide Surfaces [in Russian], Nauka, Novosibirsk (1984), p. 246.
O. Mahé, D. Frath, I. Dez, F. Marsais, V. Levacher, and J.-F. Brière, Org. Biomol. Chem., 7, 3648 (2009).
U. S. Gökşen, Y. B. Alpaslan, N. G. Kelekçi, Ş. Işık, and M. Ekizoğlu, J. Mol. Struct., 1039, 71 (2013).
V. N. Vasilev, Mycobaterioses and Pulmonary Mycoses [in Bulgarian], Medizina i Fizkultura, Sofia (1971), p. 377.
A. E. Yermakov, M. A. Uimin, V. R. Galakhov, K. Kuopper, S. Robin, and M. Neiemann, J. Metastable Nanocryst. Mater., 43, 24 (2005).
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., A64, 112 (2008).
This work was carried out with the financial support of the Grants Council of the President of the Russian Federation (grant NSh-3656-2014.3), Russian Foundation for Basic Research (grant No. 13-03-12188-ofi_m), and the Ural Branch of the Russian Academy of Sciences (projects Nos. 12-P-234-2003, 12-P-3-1030).
We express our gratitude to colleagues at the Institute of Metal Physics of the Ural Branch of the Russian Academy of Sciences, Candidate of Physical and Mathematical Sciences, Senior Scientist M. A. Uimin and Scientific Associate A. A. Mysik for providing a sample of nanosized alumina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician O. N. Chupakhin on the occasion of his 80th birthday.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 1027-1039, July, 2014.
Rights and permissions
About this article
Cite this article
Fedorova, O.V., Ovchinnikova, I.G., Kravchenko, M.A. et al. Synthesis and Tuberculostatic Activity of Pyrrolyl and Pyrazolinyl Podands. Chem Heterocycl Comp 50, 946–957 (2014). https://doi.org/10.1007/s10593-014-1549-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1549-5