Abstract
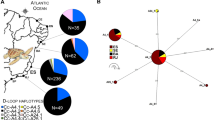
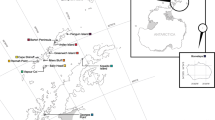
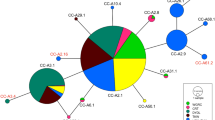
Female otariids (eared seals) frequently display strong levels of philopatry, a behaviour that has the potential to influence population structure, particularly at the mitochondrial level. Conversely, male otariids often move between breeding colonies, likely facilitating nuclear gene flow between colonies. Such gender-specific movements have the potential to influence species population structure. Here we investigate the genetic population structure of the endangered New Zealand (NZ) sea lion, using nuclear (microsatellite) and mitochondrial molecular markers, with the intention to better inform conservation through identification of management units for the species. The strong levels of female philopatry in this species have potential to lead to population structure at the mitochondrial loci. In contrast, weak or no population structure is expected across nuclear loci. NZ sea lions were sampled from the main breeding areas across the species’ current distribution (three Auckland Islands sites, two Campbell Island sites, one Stewart Island site and one Otago Peninsula site). Individuals were screened for microsatellite (n = 271; 16 loci) and mitochondrial (n = 56; 1027 bp D-loop and 1189 bp cytb). Despite a small (c. 9880 individuals) population size, moderate levels of microsatellite variation are observed in the NZ sea lions, in contrast to low levels of mitochondrial genetic variation. Results from mitochondrial DNA analyses revealed no population structure, suggesting that the strong level of female philopatry in NZ sea lions alone is not sufficient to maintain genetic population structure. Due to the frequent male movements between breeding colonies, no population structure was detected across the nuclear loci either. The absence of genetic structure suggests that, from a genetic perspective, NZ sea lions can be considered to be a single population. Despite this, the differing impacts of threats (e.g. fisheries by-catch) to each individual breeding colony must also be taken into consideration when defining management units for this endangered species.


Similar content being viewed by others
References
Acevedo-Whitehouse K, Petetti L, Duignan P, Castinel A (2009) Hookworm infection, anaemia and genetic variability of the New Zealand sea lion. Proc Biol Sci 276(1672):3523–3529
Akroyd J, Pilling G (2013) Surveillance Report Wouthern Blue Whiting Fishery. Intertek Moody Marine
Allen P, Amos W, Pomroy PP, Twiss SD (1995) Microsatellite variation in grey seals (Halichoerus grypus) shows evidence of genetic differentiation between two British breeding colonies. Mol Ecol 4:653–662
Baker CS, Chilvers BL, Constantine R, DuFresne S, Mattlin R, van Helden A, Hitchmough R (2010) Conservation status of New Zealand marine mammals (suborders Cetacea and Pinnipedia), 2009. NZ J Mar Freshwat Res 44:101–115
Bernardi G, Fain SR, Gallo-Reynoso JP, Figueroa-Carranza AL, Boeuf BJ (1998) Genetic variability in Guadalupe fur seals. J Hered 89:301–305
Berry O, Spiller LC, Campbell R, Hitchen Y, Kennington WJ (2012) Population recovery of the New Zealand fur seal in southern Australia: a molecular DNA analysis. J Mammal 93:482–490
Bickham JW, Patton JC, Loughlin TR (1996) High variability for control-region sequences in a marine mammal: implications for conservation and biogeography of Stellar sea lions (Eumetopias jubatus). J Mammal 77:95–108
Boyd IL (1991) Environmental and physiological factors controlling the reproductive cycles of pinnipeds. Can J Zool 69:1135–1148
Bradshaw CJA, Harcourt RG, Davis LS (2003) Male-biased sex ratios in New Zealand fur seal pups relative to environmental variation. Behav Ecol Sociobiol 53:1083–1085
Bradshaw CJA, Haddon M, Lonergan M (2013) Review of models and data underpinning the management of fishing-related mortality of New Zealand sea lions (Phocarctos hookeri), in the SQU6T trawl fishery. Ministry for Primary Industries, Wellington, New Zealand
Breen PA, Hilborn R, Maunder MN, Kim SW (2003) Effects of alternative control rules on the conflict between a fishery and a threatened sea lion (Phocarctos hookeri). Can J Fish Aquat Sci 60:527–541
Breen PA, Fu D, Gilbert DJ (2010) Sea lion population modelling and management procedure evaluations: Report for Project SAP2008/14, Objective 2. Presented to AEWG March 22 2010, Wellington, New Zealand
Buchanan FC, Maiers LD, Thue TD, De March BGE, Stewart REA (1998) Microsatellites from the Atlantic walrus Odobenus rosmarus rosmarus. Mol Ecol 7:1083–1085
Cameron MF, Donald BS, Proffitt KM, Garrott RA (2007) Site fidelity of Weddell seals: the effects of sex and age. Antarct Sci 19:149–155
Campbell RA, Gales NJ, Lento GM, Baker CS (2008) Islands in the sea: extreme female natal site fidelity in the Australian sea lion, Neophoca cinerea. Biol Lett 4:139–142
Chen C, Durand E, Forbes F, Francois O (2007) Bayesian clustering algorithms ascertaining spatial population structure: a new computer program and a comparison study. Mol Ecol Notes 7:747–756
Childerhouse S, Gales N (1998) Historical and modern distribution and abundance of the New Zealand sea lion Phocarctos hookeri. NZ J Zool 25:1–16
Childerhouse S, Hamer D, Maloney A, Michael S, Donnelly D, Schmitt N (2014) Preliminary report for CSP project 4522 New Zealand sea lion ground component 2013/14. New Zealand Department of Conservation.
Childerhouse S, Michael S, Adams L, Burns T, Cockburn S, Hamer D, Maloney A, Pugsley C (2015) Final Report: New Zealand sea lion research at the Auckland Islands 2014/15. Unpublished report to the Conservation Services, Blue Planet Marine, Nelson, New Zealand
Chilvers BL (2009) Foraging locations of female New Zealand sea lions (Phocarctos hookeri) from a declining colony. N Z J Ecol 33:103–116
Chilvers BL (2012) Population viability analysis of New Zealand sea lions, Auckland Islands, New Zealand’s sub-Antarctics: assessing relative impacts and uncertainty. Polar Biol 35:1607–1615
Chilvers BL (2015) Phocarctos hookeri. In: IUCN red list of threatened species (IUCN 2015), Version 2015.2. http://www.iucn-redlist.org. Accessed 30 July 2015
Chilvers BL, Wilkinson IS (2008) Philopatry and site fidelity of New Zealand sea lions (Phocarctos hookeri). Wildl Res 35:463–470
Chilvers BL, Meyer S (2017) Conservation needs for the endangered New Zealand sea lion, Phocarctos hookeri. Aquat Conserv. doi:10.1002/aqc.2742
Chilvers BL, Wilkinson IS, Childerhouse S (2007) New Zealand sea lion, Phocarctos hookeri, pup production—1995 to 2006. NZ J Mar Freshwat Res 41:205–213
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1659
Collins CJ, Rawlence NJ, Worthy TH, Scofiels RP, Tennyson AJD, Smith I, Knapp M, Waters JM (2014a) Pre-human New Zealand sea lion (Phocarctos hookeri) rookeries on mainland New Zealand. J Royal Soc NZ 44: 1–16
Collins CJ, Rawlence NJ, Knapp M, Scofield RP, Robertson BC, Smith IWG, Matisoo-Smith E, Chilvers BL, Waters JM (2014b) Extinction and recolonisation of coastal megafauna following human arrival in New Zealand. Proc Biol Sci 281:20140097
Collins CJ, Chilvers BL, Taylor M, Robertson BC (2016) Historical population size of the threatened New Zealand sea lion Phocarctos hookeri. J Mammal 97:436–443
Coltman DW, Bowen WD, Wright JM (1996) PCR primers for harbour seal (Phoca vitulina concolour) microsatellites amplify polymorphic loci in other pinniped species. Mol Ecol 5:161–163
Davis CS, Gelatt TS, Siniff D, Strobeck A (2002) Dinucleotide microsatellite markers from the Antarctic seals and their use in other Pinnipeds. Mol Ecol Notes 2:203–208
Davis CS, Stirling I, Strobeck C, Coltman DW (2008) Population structure of ice-breeding seals. Mol Ecol 17:3078–3094
de Oliveira LR, Hoffman JI, Hingst-Zaher E, Majluf P, Muelbert MMC, Morgante JS, Amos W (2007) Morphological and genetic evidence for two evolutionary significant units (ESUs) in the South American fur seal, Arctocephalus australis. Conserv Genet 9:1451–1466
de Oliveira LR, De Castro RL, Cardenas-Alayza S, Bonatto SL (2012) Conservation genetics of South American aquatic mammals: an overview of gene diversity, population strucutre, phylogeography, non-invasive methods and forensics. Mammal Rev 42:275–303
Dickerson BR, Ream RR, Vignieri SN, Bentzen P (2010) Population structure as revealed by mtDNA and microsatellites in northern fur seals, Callorhinus ursinus, throughout their range. PLoS ONE 5:e10671
DoC (2009) New Zealand sea lion species management plan: 2009–2014. Department of Conservation, Wellington, New Zealand
Drummond A, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, Field M, Heled J, Kearse M, Markowitz S et al (2011) Geneious
Earl DA, von Holdt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evolut Bioinform 1:47–50
Fay JC, Wu CI (1999) A human population bottleneck can account for the discordance between patterns of mitochondrial versus nuclear DNA variation. Mol Biol Evol 16:1003–1005
Feijoo M, Lessa EP, de Castro RL, Crespo EA (2011) Mitochondrial and microsatellite assessment of population structure of South American sea lion (Otaria flavescens) in the Southwestern Atlantic Ocean. Marine Biol 158:1857–1867
French R (2015) Campbell Island population survey of New Zealand sea lion Phocarctos hookeri. Unpublished Allan Wilson Centre summer studentship report, University of Otago, Dunedin, New Zealand
Geschke K, Chilvers BL (2009) Managing big boys: a case study on remote anaesthesia and satellite tracking of adult male New Zealand sea lions (Phocarctos hookeri). Wildl Res 36:666–674
Goldsworthy S, Francis J, Boness D, Fleischer R (2000) Variation in the mitochondrial control region in the Juan Fernandez fur seal (Arctocephalus philippii). J Hered 91:371–377
Goodman SJ (1997) Dinucleotide repeat polymorphisms at seven anonymous microsatellite loci cloned from the European harbour seal (Phoca vitulina vitulina). Anim Genet 28:310–311
Goudet J (1995) FSTAT (Version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Hernandez-Velazquez FD, Galindo-Sanchez CE, Taylor MI, de la Rosa-Velez J, Cote IM, Schramm Y, Aurioles-Gamboa D, Rico C (2005) New polymorphic microsatellite markers for California sea lions (Zalophus californianus). Mol Ecol Notes 5:140–142
Hoffman EA, Amos W (2005) Microsatellite genotyping errors: detection approaches and consequences for paternal exclusion. Mol Ecol 14:599–612
Hoffman JI, Forcada J (2012) Extreme natal site philopatry in female Antarctic fur seals. Mamm Biol 77:71–73
Hoffman EA, Matson CW, Amos M, Loughlin TR, Bickham JW (2006) Deep genetic subdivision within a continuously distributed and highly vagile marine mammal, the Stellar’s sea lion (Eumetopias jubatus). Mol Ecol 15:2821–2832
Hoffman JI, Steinfartz S, Wolf JBW (2007) Ten novel dinucleotide microsatellite loci cloned from the Galápagos sea lion (Zalophus californianus wollebaeki) are polymorphic in other pinniped species. Mol Ecol Notes 7:103–105
Jemison LA, Pendleton GW, Fritz LW, Hastings KK, Maniscalo JM, Trites AW, Gelatt TS (2013) Inter-population movements of Steller sea lions in Alaska with implications for population separation. Plos ONE 8:e70167
Jensen JL, Bohonak AJ, Kelley ST (2005) Isolation by distance, web service. BMC Genet 6:13
Lancaster ML, Arnould JPY, Kirkwood R (2010) Genetic status of an endemic marine mammal, the Australian fur seal, following historic harvesting. Anim Conserv 12:402–413
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilim A, Lopez R et al (2007) ClustalW and ClustalX version 2. Bioinformatics 23:2947–2948
Lento GM, Haddon M, Chambers GK, Baker CS (1997) Genetic variation of southern hemisphere fur seals (Arctocephalus spp.): investigation of population structure and species identity. J Hered 88:202–208
Lopes F, Hoffman JI, Valiati VH, Bonatto SL, Wolf JBW, Trillmich F, Oliveira LR (2015) Fine-scale matrilineal populaiton structure in the Galapagos fur seal and its implications for conservation management. Conserv Genet 16:1099–1113
Lynch M, Ritlan K (1999) Estimation of pairwise relatedness with molecular markers. Genetics 152:1753–1766
Majluf P, Goebel ME 1992. The capture and handling of female South American fur seals and their pups. Mar Mamm Sci 8:187–190
Maldonado JE, Davila FO, Steart BS, Geffen E, Wayne RK (1995) Intraspecific genetic differentiation in California sea lions (Zalophus californianus) from southern California and the Gulf of California. Mar Mamm Sci 11:46–58
Maloney A, Chilvers BL, Muller CG, Haley M (2012) Increasing pup production of New Zealand sea lions at Campbell Island/Motu Ihupuku: can it continue? New Zealand. J Zool 39:19–29
Manel S, Bellemain E, Swenson JE, Francois O (2004) Assumed and inferred spatial structure of populations: the Scandinavian brown bears revisited. Mol Ecol 13:1327–1331
Martínez-Cruz B, Godoy JA, Negro JJ (2004) Population genetivs after fragmentation: the case of the endangered Spanish imperial eagle (Aquila adalberti). Mol Ecol 13:2243–2255
Matthee CA, Fourie F, Oosthuizen WH, Meyer MA, Tolley KA (2006) Mitochondrial DNA sequence data of the Cape fur seal (Arctocephalus pusillus pusillus) suggest that population numbers may be affected by climatic shifts. Mar Biol 148:899–905
McConkey S, McConnell H, Lalas C, Heinrich S, Ludmerer A, McNally N, Parker E, Borofsky C, Schimanski K, McIntosh G 2002. A northward spread in the breeding distribution of the New Zealand sea lion Phocarctos hookeri. Aust Mammal 24:97–106
Meyer S, Robertson BC, Chilvers BL, Krkošek M (2015) Population dynamics reveal conservation priorities of the threatened New Zealand sea lion Phocarctos hookeri. Mar Biol 162:1587–1596
Moritz C (1994) Defining evolutionarily-significant-units for conservation. Trends Ecol Evol 9:373–375
MPI (2013) Aquatic environment and biodiversity annual review 2013. Fisheries Management Science Team, Ministry for Primary Industries, Wellington, New Zealand, 583 p
MPI (2015) New Zealand sea lion (Phocarctos hookeri). In: Aquatic environment and biodiversity annual review (2015). Pp. 18–52
Olsen MT, Andersen LW, Dietz R, Teilmann J, Härkönen T, Siegismund HR (2014) Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) Popul Manage Units 23:815–831
Osborne AJ, Zavodna M, Chilvers BL, Robertson BC, Negro SS, Kennedy MA, Gemmell NJ (2013) Extensive variation at MHC DRB in the New Zealand sea lion (Phocarctos hookeri) provides evidence for balancing selection. Heredity 111:44–56
Osborne AJ, Negro SS, Chilvers BL, Robertson BC, Kennedy MA, Gemmell NJ (2016) Genetic evidence of a population bottleneck and inbreeding in the endangered New Zealand sea lion, Phocarctos hookeri. J Hered 107:392–402
Paetkau D, Strobeck C (1994) Microsatellite analysis of genetic-variation in black bear populations. Mol Ecol 3:489–495
Palsbøll PJ, Bérubé M, Allendorf FW (2006) Identification of management units using population genetic data. Trends Ecol Evol 22:11–16
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Populaion genetic software for teaching and research-an update. Bioinformatics 28:2537–2539
Piry S, Alapetite A, Cornuet J-M, Paetkau D, Baudouin L, Estoup A (2004) GENECLASS2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539
Pomeroy PP, Twiss SD, Redman P (2000) Philopatry, site fidelity and local kin associations within Grey Seal breeding colonies. Ethology 106:899–919
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rambaut A, Drummond AJ (2009) Tracer v1.5
Raymond M, Rousset F (1995) Genepop (Version-1.2) - population-genetics software for exact tests and ecumenicism. J Hered 86:248–249
Robertson BC (2015) Comment on “Review of research and assessments on the efficacy of sea lion exclusion devices in reducing the incidental mortality of New Zealand sea lions Phocarctos hookeri in the Auckland Islands squid trawl fishery”. Fish Res 165:127–129
Robertson BC, Chilvers BL (2011) The population decline of the New Zealand sea lion Phocarctos hookeri: a review of possible causes. Mamm Rev 41:253–275
Robertson BC, Gemmell NJ (2005) Microsatellite DNA markers for the study of population structure in the New Zealand fur seal Arctocephalus forsteri. DOC Science Internal Series 196. Department of Conservation, Wellington, New Zealand
Robertson BC, Chilvers BL, Duignan PJ, Wilkinson IS, Gemmell NJ (2006) Dispersal of breeding, adult male Phocarctos hookeri: Implications for disease transmission, population management and species recovery. Biol Conserv 127:227–236
Schramm Y, Mesnick SL, de la Rosa J, Palacios DM, Lowry MS, Aurioles-Gamboa D, Snell HM, Escorza-Trevino S (2009) Phylogeography of California and Galapogas sea lions and population structure within the California sea lion. Mar Biol 156:1375–1387
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18:233–234
Smith I (1985) Sea mammal hunting and prehistoric subsistence in New Zealand. Unpublished thesis, University of Otago, Dunedin
Starke J (1986) Journal of a rambler; the journal of John Boultbee. Oxford University Press, Auckland
Taylor AC, Sherwin WB, Wayne R (1994) Genetic variation of microsatellite loci in a bottleneckd species: the norther hairy-nosed wombat Lasiorhinus krefftii. Mol Ecol 3:277–290
Tunez JI, Centron D, Cappozo HL, Cassini MH (2007) Geographic distribution and diversity of mitochondrial DNA haplotypes in South Amerivan sea lions (Otaria flavescens) and fur seals (Arctocephalus australis). Mamm Biol 72:193–203
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Walsh SP, Metzger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10:506–513
Waters JM, Epifanio JM, Gunter T, Brown BL (2000) Homing behaviour facilitates subtle genetic differentiation among river populations of Alosa sapidissima: microsatellites and mtDNA. J Fish Biol 56:622–636
Wilson GA, Rannala B (2003) Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163:1177–1191
Wolf JBW, Tautz D, Caccone A, Steinfartz S (2005) Development of new microsatellite loci and evaluation of loci from other pinniped species for the Galápogas sea lion (Zalophus californianus wollebaeki). Conserv Genet 7:461–465
Wolf JBW, Harrod C, Brunner S, Salazar S, Trillmich F, Tautz D (2008) Tracing early stages of species differentiation: ecological, morphological and genetic divergences of Galapagos sea lion populations. BMC Evol Biol 8:150
Wynen LP, Goldsworthy SD, Guiney C, Bester MN, Boyd IL, Gjertz I, Hofmeyr GJG, White RWG, Slade R (2000) Postsealing genetic variation and population structure of two species of fur seal (Arctocephalus gazella and A. tropicalis). Mol Ecol 9:299–314
Yannic G, St-Laurent M-H, Ortgeo J, Taillon J, Beauchemin A, Bernatchez L, Dussault C, Côté SD (2016) Integrating ecological and genetic structure to define management units for caribou in Eastern Canada. Conserv Genet 17:437–453
Acknowledgements
Modern P. hookeri samples were collected with funding from DoC with approval for sample collection from the DoC Animal Ethics Committee (Approvals AEC 200 2009, AEC 159, AEC 174, AEC 232). Funding for the collection of modern P. hookeri samples was provided by a New Zealand Department of Conservation Grant (DoC Inv 4219). We thank Fiona Robertson for assistance with collection of this data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Collins, C.J., Chilvers, B.L., Osborne, A. et al. Unique and isolated: population structure has implications for management of the endangered New Zealand sea lion. Conserv Genet 18, 1177–1189 (2017). https://doi.org/10.1007/s10592-017-0969-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-017-0969-z