Abstract
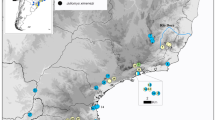
The aim of this study was to investigate whether Pleistocene climatic instability influenced the phylogeographic structure and historical demography of an endemic Atlantic Forest (AF) orchid bee, Euglossa iopoecila Dressler, which shows two main patterns of integument colors over of its geographical distribution. We based our analysis on the concatenated sequence of four mtDNA segments belonging to genes 16S (357 bp), Cytb (651 bp) and COI (1206 bp), totaling 2234 bp. Samples of E. iopoecila populations were collected in 14 AF remnants along its geographic distribution. Median-Joining haplotype networks, SAMOVA and BAPS results indicated three lineages (southern, central and northern clusters) for E. iopoecila, with two important phylogeographic ruptures. We found higher genetic diversity among samples collected in the central region of the AF, which coincides with predicted areas of climatic stability, according to recent AF stability–extinction model. The demographic analysis suggests that only the southern cluster had undergone recent population expansion, which probably started after the last glacial maximum (LGM). Our data suggest that the differentiation observed in the three mitochondrial lineages of E. iopoecila is the result of past disconnections and multiple extinction/recolonization events involving climate fluctuations. In terms of conservation, we would emphasize the importance of considering: (1) the region of the central clade as the location of the highest genetic diversity of mtDNA of E. iopoecila populations; (2) the philopatric behavior of females that tends to restrict mtDNA gene flow in particular, with direct implications for the conservation of the total genetic diversity in euglossine populations.




Similar content being viewed by others
References
Amaral FR, Albers PK, Edwards SV, Miyaki CY (2013) Multilocus tests of Pleistocene refugia and ancient divergence in a pair of Atlantic Forest antbirds (Myrmeciza). Mol Ecol 22:3996–4013
Augusto SC, Garófalo CA (2004) Nesting biology and social structure of Euglossa (Euglossa) townsendi Cockerell (Hymenoptera, Apidae, Euglossini). Insect Soc 51:400–409
Avise JC (2009) Phylogeography: retrospect and prospect. J Biogeogr 36:3–15
Batalha-Filho H, Miyaki CY (2016) Late Pleistocene divergence and postglacial expansion in the Brazilian Atlantic Forest: multilocus phylogeography of Rhopias gularis (Aves: Passeriformes). J Zool Syst Evol Res 54:137–147
Batalha-Filho H, Melo GAR, Waldschmidt AM, Campos LAO, Fernandes-Salomão TM (2009) Geographic distribution and spatial differentiation in the color pattern of abdominal stripes of the Neotropical stingless bee Melipona quadrifasciata (Hymenoptera: Apidae). Zoologia 26:213–219
Batalha-Filho H, Waldschmidt AM, Campos LAO, Tavares MG, Fernandes-Salomão TM (2010) Phylogeography and historical demography of the neotropical stingless bee Melipona quadrifasciata (Hymenoptera, Apidae): incongruence between morphology and mitochondrial DNA. Apidologie 41:534–547
Boff S, Soro A, Paxton RJ, Alves-dos-Santos I (2014) Island isolation reduces genetic diversity and connectivity but does not significantly elevate diploid male production in a neotropical orchid bee. Conserv Genet 15:1123–1135
Cabanne GS, Santos FR, Miyaki CY (2007) Phylogeography of Xiphorhynchus fuscus (Passeriformes, Dendrocolaptidae): vicariance and recent demographic expansion in southern Atlantic forest. Biol J Linn Soc 91:73–84
Cabanne GS, Trujillo-Arias N, Calderón L, D’Horta FM, Miyaki CY (2014) Phenotypic evolution of an Atlantic Forest passerine (Xiphorhynchus fuscus): biogeographic and systematic implications. Biol J Linn Soc 113:1047–1066
Cardoso DC, Cristiano MP, Tavares MG, Schubart CD, Heinze J (2015) Phylogeography of the sand dune ant Mycetophylax simplex along the Brazilian Atlantic Forest coast: remarkably low mtDNA diversity and shallow population structure. BMC Evol Biol 15:106
Carnaval AC, Moritz C (2008) Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic Forest. J Biogeogr 35:1187–1201
Carnaval AC, Hickerson MJ, Haddad CFB, Rodrigues MT, Moritz C (2009) Stability predicts genetic diversity in the Brazilian Atlantic Forest hotspot. Science 323:785–789
Corander J, Tang J (2007) Bayesian analysis of population structure based on linked molecular information. Math Biosci 205:19–31
Corander J, Marttinen P, Sirén J, Tang J (2008) Enhanced Bayesian modelling in BAPS software for learning genetic structures of populations. BMC Bioinform 9:539–553
Dellicour S, Mardulyn P (2014) Spads 1.0: a toolbox to perform spatial analyses on DNA sequence data sets. Mol Ecol Resour 14:647–651
Dick CW, Roubik DW, Gruber KF, Bermingham E (2004) Long-distance gene flow and cross-Andean dispersal of lowland rainforest bees (Apidae: Euglossini) revealed by comparative mitochondrial DNA phylogeography. Mol Ecol 13:3775–3785
Dodson CH, Dressler RL, Hills HG, Adams RM, Williams NH (1969) Biologically active compounds in orchid fragrances. Science 164:1243–1249
Dressler RL (1982) Biology of orchid bees. Annu Rev Ecol Syst 13:373–394
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214. doi:10.1186/1471-2148-7-214
Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of populations. Mol Ecol 11:2571–2581
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform 1:47–50
Faria L Jr, Melo G (2007) Species of Euglossa (Glossura) in the Brazilian Atlantic Forest, with taxonomic notes on Euglossa stellfeldi Moure (Hymenoptera, Apidae, Euglossina). Rev Bras Entomol 51:275–284
Ferrari BR, Melo GAR (2014) Deceiving colors: recognition of color morphs as separate species in orchid bees is not supported by molecular evidence. Apidologie 45:641–652
Freiria GA, Ruim JB, de Souza RF, Sofia SH (2012) Population structure and genetic diversity of the orchid bee Eufriesea violacea (Hymenoptera, Apidae, Euglossini) from Atlantic Forest remnants in southern and southeastern Brazil. Apidologie 43:392–402
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Galindo-Leal C, Câmara IG (2003) Atlantic Forest hotspot status: an overview. In: Galindo-Leal C, Câmara IG (eds) The Atlantic Forest of South America: biodiversity status, threats, and outlook. Center for Applied Biodiversity Science and Island Press, Washington, pp 3–11
Giangarelli DC, Aguiar WM, Sofia SH (2015) Orchid bee (Hymenoptera: Apidae: Euglossini) assemblages from three different threatened phytophysiognomies of the subtropical Brazilian Atlantic Forest. Apidologie 46:71–83
Haffer J (1969) Speciation in Amazonian forest birds. Science 165:131–137
Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W (2004) Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA 101:14812–14817
Heled J, Drummond AJ (2008) Bayesian inference of population size history from multiple loci. BMC Evol Biol. doi:10.1186/1471-2148-8-289
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Ho SY, Shapiro B (2011) Skyline-plot methods for estimating demographic history from nucleotide sequences. Mol Ecol Resour 11:423–434
Knowles LL, Alvarado-Serrano DF (2010) Exploring the population genetic consequences of the colonization process with spatio-temporally explicit models: insights from coupled ecological, demographic and genetic models in montane grasshoppers. Mol Ecol 19:3727–3745
Laurance WF (2009) Conserving the hottest of the hotspots. Biol Conserv 142:1137
Leigh JW, Bryant D (2015) POPART: full-feature software for haplotype network construction. Methods Ecol Evol 6:1110–1116
Leite YLR, Costa LP, Loss AC, Rocha RG, Batalha-Filho H, Bastos AC, Quaresma VS, Fagundes V, Paresque R, Passamani M, Pardini R (2016) Neotropical forest expansion during the last glacial period challenges refuge hypothesis. Proc Natl Acad Sci USA 113:1008–1013
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
López-Uribe MM, Zamudio KR, Cardoso CF, Danforth BN (2014) Climate, physiological tolerance and sex-biased dispersal shape genetic structure of Neotropical orchid bees. Mol Ecol 23:1874–1890
Michener CD (2007) The bees of the world, 2nd edn. The Johns Hopkins University Press, Baltimore
Moritz C (1994) Defining “evolutionarily significant units”. Trends Ecol Evol 9:373–375
Moure J, Melo GAR, Faria Jr L (2012) Catalogue of bees (Hymenoptera, Apoidea) in the Neotropical Region-online version. In: Euglossini Latrelli 1802. http://www.moure.cria.org.br/catalogue. Accessed 14 Apr 2016
Myers N, Mittermeier R, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nemésio A (2009) Orchid bees (Hymenoptera: Apidae) of the Brazilian Atlantic Forest. Zootaxa 2041:242
Pearson RG, Dawson TP, Liu C (2004) Modelling species distributions in Britain: a hierarchical integration of climate and land-cover data. Ecography 27:285–298
Penha RES, Gaglianone MC, Almeida FS, Boff S, Sofia SH (2015) Mitochondrial DNA of Euglossa iopoecila (Apidae, Euglossini) reveals two distinct lineages for this orchid bee species endemic to the Atlantic Forest. Apidologie 46:346–358
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Pokorny T, Loose D, Dyker G, Quezada-Euán JJG, Eltz T (2015) Dispersal ability of male orchid bees and direct evidence for long-range flights. Apidologie 46:224–237
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808
Quantum GIS Development Team (2009) Quantum GIS geographic information system. Open Source Geospatial Foundation. http://qgis.osgeo.org
Ramalho AV, Gaglianone MC, Oliveira ML (2009) Comunidades de abelhas Euglossina (Hymenoptera, Apidae) em fragmentos de Mata Atlântica no Sudeste do Brasil. Rev Bras Entomol 53:95–101
Ramírez S, Dressler RL, Ospina M (2002) Abejas euglosinas (Hymenoptera: Apidae) de la Región Neotropical: Listado de especies con notassobre su biología. Biota Colombiana 3:7–118
Ramírez SR, Roubik DW, Skov C, Pierce NE (2010) Phylogeny, diversification patterns and historical biogeography of euglossine orchid bees (Hymenoptera: Apidae). Biol J Linn Soc 100:552–572
Ramírez-Soriano A, Ramos-Onsins SE, Rozas J, Calafell F, Navarro A (2008) Statistical power analysis of neutrality tests under demographic expansions, contractions and bottlenecks with recombination. Genetics 179:555–567
Ramos-Onsins SE, Rozas J (2002) Statistical properties of new neutrality tests against population growth. Mol Biol Evol 19:2092–2100
Raymond M, Rousset F (1995) GENEPOP (Version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Ribeiro RA, Lemos-Filho JP, Ramos ACS, Lovato MB (2011) Phylogeography of the endangered rosewood Dalbergia nigra (Fabaceae): insights into the evolutionary history and conservation of the Brazilian Atlantic Forest. Heredity 106:46–57
Rocha-Filho LC, Cerântola NCM, Garófalo CA, Imperatriz-Fonseca VL, Del Lama MA (2013) Genetic differentiation of the Euglossini (Hymenoptera, Apidae) populations on a mainland coastal plain and an island in southeastern Brazil. Genetica 74:65–74
Roubik D, Hanson P (2004) Orchid bees: biology and field guide. InBio Press, Heredia
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87:651–701
SOS Mata Atlântica, Instituto Nacional de Pesquisas Espaciais (2015) Atlas dos Remanescentes florestais da Mata Atlântica, período de 2013-2014
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Thomé MTC, Zamudio KR, Giovanelli JGR, Haddad CFB, Baldissera FA Jr, Alexandrino J (2010) Phylogeography of endemic toads and post-Pliocene persistence of the Brazilian Atlantic Forest. Mol Phylogenet Evol 55:1018–1031
Van Dike F (2008) Genetic diversity—understanding conservation at genetic levels. In: Van Dike F (ed) Conservation biology: foundations, concepts, applications. Springer, Dordrecht, pp 153–184
Zimmermann Y, Roubik DW, Quezada-Euan JJG, Paxton RJ, Eltz T (2009) Single mating in orchid bees (Euglossa, Apinae): implications for mate choice and social evolution. Insect Soc 56:241–249
Zink RM, Barrowclough GF (2008) Mitochondrial DNA under siege in avian phylogeography. Mol Ecol 17:2107–2121
Acknowledgements
We would like to thank: a) CNPq and Fundação Araucária for financial support; b) CAPES for a scholarship awarded to Wilson Frantine-Silva and CNPq for the postdoctoral fellowship to Douglas C. Giangarelli; c) IBAMA/ICMBio, IAP and Instituto Florestal de São Paulo for the license for the collections; d) Reserva Natural Salto Morato (RNSM), Rebio União, Rebio Sooretama, Parque Nacional do Descobrimento, Parque Estadual Serra do Conduru, Estação Ecológica de Murici for the collecting permits; e) the staff of the State Parks of São Sebastião, Ilhabela, Ilha do Cardoso, Ubatuba (Picinguaba), RNSM and Parque Nacional do Superagui for providing infrastructure and their help in the field work; f) André Nemésio by donation of the specimens of E. iopoecila from sites BA3 and AL. Silvia H. Sofia, Maria C. Gaglianone and Isabel Alves-dos-Santos are research fellows from CNPq. We also would like to thank the reviewers for their suggestions, which substantially contributed to this paper improvement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10592_2016_905_MOESM1_ESM.jpg
Supplementary material 1 (JPEG 277 kb). Figure S1. a) Relationship between the genetic distances measured as F ST/(1 − F ST) and the logarithm of geographical distance (km); b) genetic distances (F ST) and geographic distance (km) between pairs of Euglossa iopoecila samples surveyed across the Brazilian Atlantic Forest. Minimum distance was around 2 km (SP3-SP4) and maximum distance was 2341 km (SC- AL)
Rights and permissions
About this article
Cite this article
Frantine-Silva, W., Giangarelli, D.C., Penha, R.E.S. et al. Phylogeography and historical demography of the orchid bee Euglossa iopoecila: signs of vicariant events associated to Quaternary climatic changes. Conserv Genet 18, 539–552 (2017). https://doi.org/10.1007/s10592-016-0905-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-016-0905-7