Abstract
Galium cracoviense is a narrow endemic diploid species with a highly restricted natural range of occurrence, confined to a system of limestone outcrops in an area covering ca. 25 km2 in southern Poland. It is an excellent example of a naturally very rare species which most probably has persisted in the same area since its divergence. We used the first exhaustive inventory of its subpopulations, range-wide fine-scale sampling and AFLP fingerprinting to examine the genetic structure of G. cracoviense. The aims of the study were to verify whether this ‘old rare’ species maintains high levels of genetic variation despite low population resources; to infer from the spatial pattern of genetic relatedness of individuals any limitations on dispersal and gene flow that may contribute to maintenance of its highly restricted range; and to place our results in the context of conservation strategies for G. cracoviense. Based on AMOVA and PCoA we found high genetic variation (80 % polymorphic markers) and weak divergence among subpopulations, despite the spatial isolation of subpopulations (ΦST = 0.11). The high genetic variation found in most subpopulations makes them equally important reservoirs of genetic diversity. In view of the microspatial genetic structure of the subpopulations revealed by autocorrelation analyses, we recommend that if plant material or seeds of this species are collected for ex situ conservation, multiple sampling of neighboring plants (distances of the first lag in autocorrelation analysis) should be avoided so that the largest range of diversity can be covered by sampling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When assessing the risk of species extinction for conservation purposes it is important to have knowledge of the dependence between the geographic distribution of populations and their level of genetic variation and diversity, due to the possibility of genetic depauperation associated with often small population sizes and/or the influence of specific habitat requirements. It has been shown that small plant populations often face increased inbreeding depression and reduced genetic variation as a result of genetic drift (Ellstrand and Elam 1993; Young et al. 1996; Frankham 2005; Grady et al. 2006). In both rare and widespread species, range fragmentation often has detrimental consequences for the genetic diversity of populations (Honnay and Jacquemyn 2007). A major group of rare taxa is composed of species considered rare today but which were much more common in a particular place in the past. The decline of their population number and size and the increase of their isolation are typically due to human influences (e.g. direct or indirect habitat disturbance). Such taxa, described as ‘new rare species’ (Huenneke 1991), are often endangered by genetic erosion (Ouborg and van Treuren 1994). The observed strong effects on genetic diversity in this group may result from a recent (i.e., in non-evolutionary time) drop in the regional abundance of populations (Huenneke 1991; Gitzendanner and Soltis 2000).
Some rare species, described as ‘naturally rare species’ (or ‘old rare species’), are inherently associated with specific and rare habitats or with a peculiar geographic location such as an island, mountain range or other geographically well-delimited area. They form small, restricted and naturally isolated populations over a long historical period (Holderegger 1997; Pegtel 1998). Naturally rare species include such groups as narrow endemics and relicts (Pegtel 1998). Such species, characterized by stenotopic ecology and a naturally fragmented distribution, are generally expected to have populations showing higher differentiation and lower variation than in widespread species (Gitzendanner and Soltis 2000; Cole 2003). On the other hand, there are several examples of species which, despite their rarity, retain a relatively high level of genetic variability and low interpopulation differentiation, indicating that they are well adapted to such a spatial distribution (e.g. Ligularia sibirica, Šmídová et al. 2011; Saxifraga paniculata, Reisch et al. 2003). Studies of this group of species can help improve our understanding of the effects of random genetic processes on the genetic structure of populations and on the survival and evolutionary potential of small isolated populations in nature (Schmidt and Jensen 2000; Escaravage et al. 2011).
In this study we assessed the genetic structure of a naturally rare species, Galium cracoviense Ehrend. (Rubiaceae), listed on the global IUCN Red List of Threatened Plants (Walter and Gillett 1998), in Annex I to the Convention on the Conservation of European Wildlife and Natural Habitats (Bern Convention), and in Annex II to the European Habitats Directive. It furnishes a good case study thanks to its clearly defined and extremely small distribution range, coupled with the strong likelihood that it represents an instance of long-term, historical persistence of a distribution typical of an old rare species. The highly restricted area of G. cracoviense may be a natural consequence of its specific habitat requirements and the historical conditions of the Pleistocene glaciations in the region. That is why it is explicitly treated as an old rare species (Cieślak and Szeląg 2009). It grows on calcareous rock on the northern margin of the Wyżyna Krakowsko-Częstochowska upland in southern Poland (Kucowa 1967). The habitat characteristics and the glaciation history of the area where it occurs support the view that this species has persisted in the same area since its divergence (Cieślak and Szeląg 2010). Literature data do not document any recent (anthropogenic) loss of parts of its range (Kucowa 1967; Walusiak 2010). The very restricted occurrence of G. cracoviense enabled us to precisely define its total population resources as well as to sample its whole range of distribution and thus the whole extent of its genetic variation. A high and uniform level of genetic variation was earlier recorded in a pilot study of three subpopulations of G. cracoviense (Cieślak and Szeląg 2009). Since the distribution of G. cracoviense has never been mapped systematically before, our first aim was to inventory the subpopulations in detail over its entire area. Then we assessed the level of genetic variation and the genetic structure in all significant, spatially delimited subpopulations of G. cracoviense within its range, using amplified fragment length polymorphism (AFLP) fingerprinting (Vos et al. 1995).
In particular, we wanted to test whether an old rare species maintains high genetic variation despite its low population resources. We also wanted to understand the spatial pattern of genetic relatedness of individuals; a finding of sharp divergence among groups might point to limitations on dispersal and gene flow as factors maintaining its very restricted species range. Finally, we considered the implications of the population’s genetic structure for strategies to protect G. cracoviense.
Materials and methods
Biology and habitat of Galium cracoviense
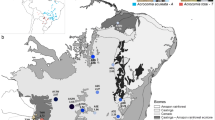
Galium cracoviense is a perennial herbaceous plant which forms clumps, produces numerous shoots 5–10 cm long, and flowers profusely in May and June (Kucowa 1967). A single clump consists of one individual having ca. 250–300 flowers; whole clumps bloom, and almost all the flowers produce fruit. A single flower produces one schizocarp bearing two seeds. It is insect-pollinated and reproduces both vegetatively and generatively (Mirek 2004). It is a diploid species (2n = 22; Piotrowicz 1958). Its range is limited to a few hills (max. 514 m a.s.l.) surrounding Olsztyn village (Fig. 1). It grows in crevices of calcareous rock and rocky outcrops of Jurassic age in the Festucetum pallentis community and rarely on scree at the base of cliffs. Its subpopulation areas vary from 2 m2 (Skałki Duże) to ca. 4000 m2 (Góra Zamkowa). Subpopulation sizes range from single clumps (Łysa Góra, Skałki Duże) to compact groups (Góra Zamkowa, Wzgórze Brodła) (Walusiak 2010).
Distribution range of all Galium cracoviense subpopulations. Subpopulation codes as in Table 1. 1 built-up area, 2 Galium cracoviense subpopulation, 3 woodland, 4 road
The species grows and fruits abundantly on both northern and southern slopes. It is resistant to spring frost and begins vegetative growth in early March (Z. Szeląg, personal observations). It winters, with dried aboveground parts, in places under thick snow cover but also in crevices of bare rock directly exposed to frost. It tolerates summer rainfall deficits. When autumn begins in late September/early October it produces numerous vegetative shoots.
Plant material
The material was collected from the entire occurrence area of Galium cracoviense, as inventoried in detail in the first step of the present study (Fig. 1). Subpopulations were defined as groups of plants well delimited on the landscape. Each subpopulation was sampled evenly and the number of samples was adjusted to the size of the subpopulation (between 10 and 30 samples per subpopulation). In total, 200 samples from eight subpopulations (formed of more than three individual patches) were sampled during one vegetative season (Table 1). At each locality, samples were collected on transects established along as horizontal a line as possible. Vertically the transects spanned a few meters depending on landform features.
AFLP procedure
AFLP analysis generally followed the procedure of Vos et al. (1995), as described in detail by Cieślak et al. (2007). Preselective and selective primers were used according to Cieślak and Szeląg (2009). Genotyping reproducibility was tested by including intra- and interplate duplicates of ca. 5 % of the samples (Bonin et al. 2007); reproducibility in our AFLP analyses was 96 %.
Data analysis
The AFLP profiles were analyzed using Genographer 1.6.0 (http://hordeum.oscs.montana.edu/genographer), which was applied to read fragments in the 50–500 bp range and to code scores into a binary absence/presence (0/1) matrix.
The number of polymorphic bands and the expected heterozygosity index (Hj) (Nei 1978) were calculated for the whole species and for each subpopulation separately (also in relation to all bands, %poly). Private bands (Mp) and discriminating bands (Md) (Cieślak et al. 2007) were checked for the subpopulations.
Relationships between individuals were analyzed based on a Nei and Li (1979) distance matrix and tested by principal coordinates analysis (PCoA) computed using FAMD 1.108 beta (Schlüter and Harris 2006) and the neighbor-joining method implemented in SPLITStree 4.10 (Huson and Bryant 2006). Support for the branches was estimated by bootstrapping (105 replicates). Isolation by distance was estimated from pairwise estimates of the FST/(1 − FST) ratio (ARLEQUIN v. 3.01; Excoffier et al. 2005) and log geographic distance (natural logarithm scale).
The nonparametric Spearman rank test was used to check for correlations between subpopulation size and the number of polymorphic bands and Hj values. Bayesian analyses were carried out with STRUCTURE 2.2 (Pritchard et al. 2000; Falush et al. 2007). The AFLP data set was converted with the AFLPdat script (Ehrich 2006) with the option for dominant markers (Falush et al. 2007). The analyses were performed using an admixture model with correlated allele frequencies, 106 iterations and a burn-in of 2 × 105 iterations. Ten replicates for each K were analyzed for K = 1–10. The numbers of groups were chosen after the STRUCTURE output files were analyzed in R software (R Development Core Team 2004) using the Structure.sum script (D. Ehrich; available from http://www.nhm.uio.no/ncb/) to visualize the estimated likelihood of each run, the coefficient of similarity between runs, and Evanno’s ΔK (Evanno et al. 2005). In the first place the ΔK measure was assessed to identify the appropriate number of clusters of individuals. The following parameters were further used to select the correct number of groups: lnP(D) values, estimates of posterior probabilities examined as a function of increasing K, consistency of probability and grouping over multiple runs, and the absence of empty groups. Analyses using STRUCTURE 2.2 were done on the Bioportal at the University of Oslo.
The distribution of genetic variation was examined by analysis of molecular variance (AMOVA) as implemented in GENALEX 6.41 (Peakall and Smouse 2006), which partitions total genetic variation based on pairwise squared Euclidean distance within versus among subpopulations. F-statistics were computed under the random mating hypothesis with ARLEQUIN 3.01 (Excoffier et al. 2005). This provided the unbiased FST estimator, ΦST, following Weir and Cockerham (1984), for which 95 % confidence intervals were obtained by bootstrapping 1000 replicates over loci.
For each subpopulation with more than ten individuals an autocorrelation analysis was performed. It used the binary matrix of the AFLP profiles and a spatial coordinate matrix of all sampled individuals. We performed the analysis with 5–9 distance classes but present only seven distance classes as a compromise between sample size per class and the physical distance covered per class. Kinship coefficients (Fj) were computed as described by Hardy and Vekemans (2002), averaged for each distance class (on a logarithmic scale), and their standard errors were obtained by jackknifing over loci. It should be mentioned here that according to Cavers et al. (2005), for example, our sampling strategy for some subpopulations does not fulfill standard requirements, since sample sizes within subpopulations are too low for reliable results to be obtained (see Table 1). On the other hand, however, all analyzed individuals were on linear transects (corresponding to subpopulation shape); that is, they were on strips several meters wide and several hundred meters long. Since assessing population structure requires less dense sampling for a one-dimensional area than for the two-dimensional areas discussed by Cavers et al. (2005), it seems justified to ease the sampling requirement; also justifying our strategy is the compatibility of the results we obtained for the two least numerous subpopulations (F and H) and for the remaining ones, discussed later.
We estimated the regression slopes of kinship coefficients on the natural logarithm of geographic distances between pairs of individuals (hereinafter, ‘slope’). Between-location permutations of individuals were used to check the significance of kinship coefficients (Hardy 2003) and regression slopes (5000 permutations). We calculated parameter Sp (Vekemans and Hardy 2004) to characterize the strength of genetic structure, which is independent of the sampling scheme and thus allows comparison between studies. All spatial autocorrelation analyses were performed with SPAGeDi 1.3 (Hardy and Vekemans 2002). To estimate the amount of biparental inbreeding we used the approach proposed by Vekemans and Hardy (2004), and we assumed that the maximum estimate of biparental inbreeding areas is equal in value to the point on the axis where the correlogram curve for the subpopulation crosses this axis.
Results
Species analysis
We analyzed 200 samples of Galium cracoviense and obtained 157 polymorphic bands (80 % of all bands). The number of polymorphic bands in the subpopulations ranged from 97 (62.58 %, pop. G) to 121 (78.06 %, pop. B); the mean number per subpopulation was 112 (SD = 7.59). Private or discriminating (fixed) bands were not recorded from any of the subpopulations. Expected heterozygosity (Hj) ranged from 0.21 (pop. G) to 0.27 (pops B and C) and reached 0.24 (SD = 0.17) on average. The nonparametric Spearman test did not find any significant relationship between subpopulation size and number of polymorphic bands (p < 0.09) nor Hj (p < 0.09).
AMOVA at species level showed that variation within subpopulations significantly exceeded variation between subpopulations: 90.98 and 9.02 %, respectively. Dominance of intrapopulation variation over variation between subpopulations and between groups also appeared in analyses incorporating the genetic groups identified in STRUCTURE: 88.56 %, 11.30 % (p < 0.001) and 0.15 % (p < 0.38), respectively. ΦST values were substantially similar (0.09 and 0.11, p < 0.001), indicating high similarity among the G. cracoviense subpopulations. A comparison of the ΦST values between subpopulation pairs, ranging from 0.03 (between B and D) to 0.13 (between G and H), also supported the prevailing homogeneity of genetic variation across subpopulations. FST values differed significantly from zero.
The test of isolation by distance did not indicate such isolation, since the pairwise genetic distances measured as FST/(1 − FST) versus log geographic distance between pairs of subpopulations were not found to be dependent; the regression line is described by \({\text{y}} = 0.0013\left( { \pm 0.0136} \right){\text{x}} + 0.1219\left( { \pm 0.0096} \right)\) (Fig. 2). Neighbor-joining analysis failed to support spatial clustering. No groups (neither subpopulation nor geographic) were significantly supported (with at least moderate bootstrap support over 50 %; data not shown), which also indicates high genetic variation and a lack of divergence within species.
In PCoA analyses the individuals formed a dense, homogenous complex along axes 1 and 2 (6.25 and 4.43 %, respectively) but the specimens within the complex displayed a partial correlation with the geographic location of the subpopulations to which they belong (Fig. 3).
Ordination of individual AFLP profiles of Galium cracoviense in principal coordinate analysis (PCoA), A first two vectors and B second and third vectors. Individuals are labeled with subpopulation codes as in Table 1
The optimal division within G. cracoviense was found at K = 2 in the STRUCTURE Bayesian analysis (as indicated by ΔK values). However, the genetic groups were not spatially segregated by subpopulations and their contribution to individual subpopulations varied. One genetic group tended to dominate in all the subpopulations; the other was present to a lesser degree. The reverse was the case only for subpopulation D (Fig. 4).
Results of STRUCTURE analysis for Galium cracoviense. Bar graph of subpopulation assignment of individuals for K = 2. Subpopulations are separated by vertical lines, and each individual is represented by a thin bar. Subpopulation codes as in Table 1
Population analysis
Spatial autocorrelation functions (SAFs) were determined for each subpopulation separately (Fig. 5). The analysis was performed for seven distance intervals, making the same number of comparisons for each interval. Kinship coefficients (Fij) describing the degree of relatedness between specimen pairs as a function of distance exhibited a decreasing tendency in each case. The highest positive values (0.04–0.06) were recorded for the first lag. In all subpopulations examined, the degree of genetic similarity was comparable for all individuals growing close together. For each subpopulation we obtained a negative slope of the regression line describing the relationship Fij(m). Three types of dependence curves were identified based on the shape of the SAF. Type one is characteristic of subpopulations A and E, showing a continuous monotonic decrease of Fij values with the increase of distance between specimens. Type two is characteristic of subpopulations B, C, F and H, showing a rapid drop of Fij to negative values, then an increase, leveling near zero. The third type, intermediate, was noted for subpopulation D only.
Spatial autocorrelation analysis of Galium cracoviense subpopulations. Kinship coefficients are plotted against geographic distance (average distance between all pairs of individuals in a given distance class). Subpopulation codes as in Table 1
If the intersection between the SAF and the distance axis is a measure of the size of the occurrence area of the most genetically similar specimens, such areas are considerably larger for subpopulations A and E (ca. 59 and 79 m, respectively) than for the other subpopulations: 14 m for B, 23 m for C, 14 m for D, and 13 m for H. This does not mean that these areas are more or less homogeneous as one unit, but only that the distribution of genetically similar individuals forms a structure within them. The Sp coefficients for the subpopulations ranged from Sp = 0.043 (pop. A) to Sp = 0.009 (pop. B), the mean for G. cracoviense being Sp = 0.022 (SD = 0.011).
Discussion
Endemic and narrowly distributed plants with an island distribution usually show lower genetic diversity and higher genetic variability than their relatives having wider distribution areas (Hamrick and Godt 1996). In Galium cracoviense we found high genetic diversity and low internal divergence despite the small total distribution range and the clear spatial delimitation of subpopulations. Over 80 % of the markers were polymorphic (with much more than 60 % polymorphic markers in the least variable of the subpopulations), reflected in high Hj values (0.24 on average). This conclusion, based on comprehensive sampling, remains in line with an earlier pilot study (Cieślak and Szeląg 2009). Direct comparison of our results with other studies is problematic, since genetic diversity depends on numerous factors such as life-history traits, geographic range, and even the type of molecular method used (Nybom 2004). Nevertheless, in the group of endemic taxa of rocky/mountainous habitats we find several examples of species characterized by high genetic variability, for example Aster pyrenaeus (Escaravage et al. 2011), Astragalus albens (Neel 2008), Campanula sabatia (Federica et al. 2012) or representatives of the genus Opisthopappus (Guo et al. 2013). Further examples of species with high levels of genetic variability in the population are found in more widespread alpine species having topographically isolated populations inhabiting a harsh environment, such as Eryngium alpinum L. (Hj = 0.20; Gaudeul et al. 2000), Epilobium fleischeri Hochst., Geum reptans L. and Campanula thyrsoides L. (Hj = 0.19, 0.21 and 0.20, respectively; Kuss et al. 2008), or Senecio boissieri DC (Hj = 0.19, Peredo et al. 2009). High genetic variation and high reproductive capacity characterize the isolated, relict populations of calciphilous species persisting in limestone rock habitats in Central Europe (Reisch et al. 2003; Vogler and Reisch 2013). Within-population diversity is influenced by the life-history traits of the species, such as the type of breeding system. For vascular plant populations the breeding system can strongly affect the extant genetic variation (Holsinger and Steinbachs 1997; Schneller and Holderegger 1996). Unfortunately, no direct experimental assessment of the breeding system of Galium cracoviense is available. It is assumed that the species reproduces vegetatively through rhizomes and, in parallel, generatively by seeds. Generally, higher genetic variation within populations has been noted in outcrossing species, whereas populations of selfing species or species with a mixed mating system are often less variable genetically (Hamrick and Godt 1996; Nybom 2004). Based on the high genetic variation we observed within subpopulations (80–84 %) in our study, we suspect that outcrossing is likely an important factor maintaining the genetic properties of the subpopulations.
The genetic differentiation we found among G. cracoviense subpopulations is characterized by a ΦST value of 0.11. It is lower than the average reported for endemic plant species (ΦST = 0.26, Nybom 2004), and is moderate to low as compared against the range of values obtained for endemic plants using AFLP fingerprinting: for example, Medicago citrina, ΦST = 0.18 − 0.55 (Juan et al. 2004), Hieracium eriophorum, ΦST = 0.09 (Frey et al. 2012) or Astragalus albens, ΦST = 0.01 (Neel 2008). The rather low ΦST value may reflect, in part, the small size of the overall distribution area (Reisch and Bernhardt-Römermann 2014).
The low genetic divergence and relatively high Hj values found for G. cracoviense are likely the result of regular interpopulation pollen flow coupled with long life span and the co-occurrence of individuals representing different generations. The lack of correlation between geographic and genetic distance (Fig. 2) and the homogeneity of genetic variation may indicate that historically the population abundance of this species has not fallen below the critical threshold that would trigger processes of genetic depauperation. Thus, G. cracoviense represents the group of rare species having a highly restricted distribution and specific habitat requirements, which are able to maintain high genetic variation and gene flow, thereby preventing strong differentiation. It seems that genetic factors are not likely to be the main factors that maintain the extremely restricted geographic range of this species.
The operation of gene flow among subpopulations is mostly supported by the spatial distribution of the two main groups disclosed by STRUCTURE analysis (dominance of one genetic group but balanced participation of the second group across subpopulations). On the other hand, it is difficult to identify the reasons that led to the contribution of groups in subpopulation D, opposite to that of the rest of the subpopulations (Fig. 4). This pattern might be treated as a relict of a rare lineage maintained by long-term limited gene exchange, but this does not seem very plausible in view of the generally low genetic isolation between subpopulations. The pattern may be a result of a drastic reduction of population size, possibly the outcome of a stochastic event, with the edge position of subpopulation D in the distribution area additionally limiting gene flow between this subpopulation and other subpopulations in the range (although this is not clearly reflected by the current pairwise ΦST values). It is also worth noting that the individuals usually showed little admixture, in most cases belonging to one or another of the genetic groups. In consequence, the genetic groups were not homogeneously distributed across subpopulations but were grouped, especially for subpopulation D (Fig. 4). This may suggest an interplay of factors including (i) spatially restricted seed dispersal, generating groups of higher kinship (see also below), and (ii) a higher than expected contribution of vegetative growth, coupled with long life span of individuals.
Additionally, some human influence on the genetic structure, altering natural dispersal patterns, cannot be ruled out, because for a long time in the past the immediate area was a site of limestone quarrying as well as sheep grazing, and today it is a hiking area.
Although the general test did not indicate significant isolation by distance, a certain spatial structure was suggested by autocorrelation analyses within subpopulations (Fig. 5). In Galium cracoviense, a barochoric species, the range of seed dispersal may be the chief factor limiting gene flow. This is supported by the Sp coefficients calculated for the subpopulations, which are comparable with the values found for gravity-dispersed species (Vekemans and Hardy 2004). Propagules reach the ground towards the base of the rock wall by gravity, mostly close to the mother plants. They may be stopped at some small distance, mostly as they roll down or encounter an obstacle such as a rock. The range of such seed dispersal is short, and individuals form groups of higher kinship (as shown in PCoA, Fig. 3). Furthermore, the availability of suitable sites for seed germination and plant development may also shape the local population structure (Sokal et al. 1989). In the network of crevices in cliffs there are few places where a root system can be established. Analysis of the SAFs showed areas with higher genetic similarity of individuals at the subpopulation level. The relatively low individual-level admixture in STRUCTURE and the mostly clumped distribution of individuals representing genetic groups (Fig. 4) support the conclusions drawn above from the analysis of Fij values versus distance presented in Fig. 5. On the other hand, on this terrain seeds can sometimes be dispersed over greater distances by local gusts. Such long-distance dispersal events lead to gene flow between regions and prevent the genetic isolation of subpopulations from increasing (cf. Crema et al. 2009).
Galium cracoviense clearly is a rare species that maintains high within-population genetic variation and high connectivity among its subpopulations. However, despite its small distribution range there is some spatial segregation of genetic variation. Both facets of its reproduction may influence the genetic structure at various spatial scales by, for example, increasing the segregation of genetic groups in subpopulations through vegetative growth, and counteracting strong differentiation through pollen-mediated gene flow and long-distance seed dispersal events. From the conservation point of view, the genetic structure of the G. cracoviense population did not show any clearly divergent units that could serve as the basis for designating priority conservation units. The high genetic variation in most subpopulations makes them equally important reservoirs of genetic diversity. In view of the microspatial genetic structure revealed in the subpopulations, we recommend that if plant material or seeds of this species are collected for ex situ conservation, multiple sampling of neighboring plants (distances of the first lag in autocorrelation analysis) should be avoided so that the largest range of diversity can be covered by sampling. Finally we draw attention to subpopulation D, the only one showing the opposite proportion of genetic groups; this curious pattern remains to be explained.
References
Bonin A, Ehrich D, Manel S (2007) Statistical analysis of amplified fragment length polymorphism data: a toolbox for molecular ecologists and evolutionists. Mol Ecol 16:3737–3758
Cavers S, Degen B, Caron H, Lemes MR, Margis R, Salgueiro F, Lowe AJ (2005) Optimal sampling strategy for estimation of spatial genetic structure in tree populations. Heredity 95:281–289
Cieślak E, Szeląg Z (2009) Genetic diversity of Galium cracoviense (Rubiaceae)—the Polish endemic plant. Acta Soc Bot Poloniae 78(2):123–129
Cieślak E, Szeląg Z (2010) Genetic diversity of Galium cracoviense, G. oelandicum and G. sudeticum (Rubiaceae). Acta Soc Bot Poloniae 79(4):269–275
Cieślak E, Ronikier M, Koch MA (2007) Western Ukrainian Cochlearia (Brassicaceae)—the identity of an isolated edge population. Taxon 56(1):112–118
Cole CT (2003) Genetic variation in rare and common plants. Annu Rev Ecol Syst 34:213–237
Crema S, Cristofolini G, Rossi M (2009) Conte L (2009) High genetic diversity detected in the endemic Primula apennina Widmer (Primulaceae) using ISSR fingerprinting. Plant Syst Evol 280:29–36
Ehrich D (2006) AFLPdat: a collection of R functions for convenient handling of AFLP data. Mol Ecol Notes 6:603–604
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Evol Syst 24:217–242
Escaravage N, Cambeccdes J, Largier G, Pornon A (2011) Conservation genetics of the rare Pyreneo-Cantabrian endemic Aster pyrenaeus (Asteraceae). AoB PLANTS 2011, plr029
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578
Federica N, Benedetti L, Airò M, Ruffoni B, Mercuri A, Minuto L, Casazza G (2012) Spatial Genetic structure of Campanula sabatia, a threatened narrow endemic species of the Mediterranean Basin. Folia Geobot 47:249–262
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Frey D, Haag CR, Kozlowski G, Tison JM, Mráz P (2012) High genetic and morphological diversity despite range contraction in the diploid Hieracium eriophorum (Asteraceae) endemic to the coastal sand dunes of south-west France. Bot J Linn Soc 169:365–377
Gaudeul M, Taberlet P, Till-Bottraud I (2000) Genetic diversity in an endangered alpine plant, Eryngium alpinum L. (Apiaceae), inferred from amplified fragment length polymorphism markers. Mol Ecol 9(10):1625–1637
Gitzendanner MA, Soltis PS (2000) Patterns of genetic variation in rare and widespread plant congeners. Am J Bot 87:783–792
Guo R, Zhou L, Zhao H, Chen F (2013) High genetic diversity and insignificant interspecific differentiation on Opisthopappus Shih, an endangered cliff genus endemic to the Taihang Mountains of China. Sci World J 3(275753)
Grady JJ, O’ Brook BW, Reed DH, Ballou JD, Tonkyn DW, Frankham R (2006) Realistic levels of inbreeding depression strongly affect extinction risk in wild populations. Biol Conserv 33(1):42–51
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos Trans R Soc Lond [Biol] 351:1291–1298
Hardy OJ (2003) Estimation of pair wise relatedness between individuals and characterization of isolation by distance processes using dominant genetic markers. Mol Ecol 12:1577–1588
Hardy OJ, Vekemans X (2002) SPAGeDi: a versatile computer program to analyze spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620 [SPAGeDi software, http://www.ulb.ac.be/sciences/ecoevol/spagedi.html]
Holderegger R (1997) Recent perspectives in conservation biology of rare plants. Bull Geobot Inst ETH Zur 63:109–116
Holsinger KE, Steinbachs JE (1997) Mating systems and evolution in flowering plants. In: Iwatsuki K, Raven PH (eds) Evolution and diversification of land plants. Springer, Tokyo, pp 223–248
Honnay O, Jacquemyn H (2007) Susceptibility of common and rare plant species to the genetic consequences of habitat fragmentation. Conserv Biol 21:823–831
Huenneke LF (1991) Ecological implications of genetic variation in plant population. In: Falk DA, Holsinger KE (eds) Genetic and conservation of rare species. Oxford University Press, New York, pp 31–44
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23(2):254–267
Juan A, Crespo MB, Cowan RS, Lexer C, Fay MF (2004) Patterns of variability and gene flow in Medicago citrina, an endangered endemic of islands in the western Mediterranean, as revealed by amplified fragment length polymorphism (AFLP). Mol Ecol 13(9):2679–2690
Kucowa I (1967) Galium cracoviense Ehrendf. In: Pawłowski B (ed) Flora Polska. Rośliny Naczyniowe Polski i Ziem Ościennych. T. XI. Państwowe Wydawnictwo Naukowe, Warszawa, pp 313–314
Kuss P, Pluess AR, Ægisdóttir HH, Stöcklin J (2008) Spatial isolation and genetic differentiation in naturally fragmented plant populations of the Swiss Alps. J Plant Ecol 1(3):149–159
Mirek Z (2004) Galium cracoviense Ehrend. Przytulia krakowska, Przytulia malopolska. Poradnik i ochrony siedlisk i gatunków. Natura 2000—podręcznik metodyczny. T. 9. Ministerstwo Środowiska, Wraszawa, pp 125–127
Neel MC (2008) Patch connectivity and genetic diversity conservation in the federally endangered and narrowly endemic plant species Astragalus albens (Fabaceae). Biol Conserv 141:939–955
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13(5):1143–1155
Ouborg NJ, van Treuren R (1994) The significance of genetic erosion in the process of extinction IV. Inbreeding load and heterosis in relation to population size in the mint Salvia pratensis. Evolution 48:996–1008
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pegtel MD (1998) Rare vascular plant species at risk: recovery by seeding? Appl Veg Sci 1:67–74
Peredo EL, Revilla MÁ, Jiménez-Alfaro B, Bueno Á, Prieto JAF, Abbott RJ (2009) Historical biogeography of a disjunctly distributed, Spanish alpine plant, Senecio boissieri (Asteraceae). Taxon 58(3):883–892
Piotrowicz M (1958) Caryological studies in some species of the genus Galium L. Acta Biol Crac Ser Bot 1:159–169
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Development Core Team (2004). R: a language and enviroment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.r-project.org
Reisch C, Bernhardt-Römermann M (2014) The impact of study design and life history traits on genetic variation of plants determined with AFLP’s. Plant Ecol 215:1493–1511
Reisch C, Poschlod P, Wingender R (2003) Genetic variation of Saxifraga paniculata Mill. (Saxifragaceae): molecular evidence for glacial relict endemism in central Europe. Biol J Linn Soc 8(1):11–21
Schlüter PM, Harris SA (2006) Analysis of multilocus fingerprinting data sets containing missing data. Mol Ecol Notes 6:569–572
Schmidt K, Jensen K (2000) Genetic structure and AFLP variation of remnant populations in the rare plant Pedicularis palustris (Scrophulariaceae) and its relation to population size and reproductive components. Am J Bot 87(5):678–689
Schneller JJ, Holderegger R (1996) Genetic variation in small, isolated fern populations. J Veg Sci 7:113–120
Šmídová A, Münzbergová Z, Placková I (2011) Genetic diversity of a relict plant species, Ligularia sibirica (L.) Cass. (Asteraceae). Flora 206(2):151–157
Sokal RR, Jacquez GM, Wooten MC (1989) Spatial autocorrelation analysis of migration and selection. Genetics 121:845–855
Vekemans X, Hardy OJ (2004) New insights from fine-scale spatial genetic structure analyses in plant populations. Mol Ecol 13:921–935
Vogler F, Reisch Ch (2013) Vital survivors: low genetic variation but high germination in glacial relict populations of the typical rock plant Draba aizoides. Biodivers Conserv 22:1301–1316
Vos P, Hogers R, Bleeker R, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucl Acid Res 23:4407–4414
Walter KS, Gillett HJ (eds) (1998) 1997 IUCN red list of threatened plants. Cambridge, IUCN, World Conservation Union, p 506
Walusiak E (2010) Przytulia krakowska Galium cracoviense Ehrend. In: Perzanowska J (ed) Monitoring gatunków roślin. Przewodnik metodyczny. GIOŚ, Warszawa, pp 34–47
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38(6):1358–1370
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418
Acknowledgments
We are indebted to the Editor, Alistair Jump, and three anonymous reviewers for their constructive and insightful comments. Michael Jacobs line-edited the paper for submission. The study was supported in part by statutory funds of the Institute of Botany, Polish Academy of Sciences as well as Ministry of Science and Higher Education, Warsaw, Poland.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Cieślak, E., Cieślak, J., Szeląg, Z. et al. Genetic structure of Galium cracoviense (Rubiaceae): a naturally rare species with an extremely small distribution range. Conserv Genet 16, 929–938 (2015). https://doi.org/10.1007/s10592-015-0711-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-015-0711-7