Abstract
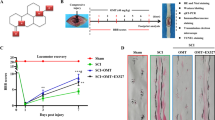
Spinal cord injury (SCI) is a devastating neurological disorder. Autophagy is induced and plays a crucial role in SCI. Ginsenoside Rb1 (Rb1), one of the major active components extracted from Panax Ginseng CA Meyer, has exhibited neuroprotective effects in various neurodegenerative diseases. However, it remains unknown whether autophagy is involved in the neuroprotection of Rb1 on SCI. In this study, we examined the regulation of autophagy following Rb1 treatment and its involvement in the Rb1-induced neuroprotection in SCI and in vitro injury model. Firstly, we found that Rb1 treatment decreased the loss of motor neurons and promoted function recovery in the SCI model. Furthermore, we found that Rb1 treatment inhibited autophagy in neurons, and suppressed neuronal apoptosis and autophagic cell death in the SCI model. Finally, in the in vitro injury model, Rb1 treatment increased the viability of PC12 cells and suppressed apoptosis by inhibiting excessive autophagy, whereas stimulation of autophagy by rapamycin abolished the anti-apoptosis effect of Rb1. Taken together, these findings suggest that the inhibition of autophagy is involved in the neuroprotective effects of Rb1 on SCI.






Similar content being viewed by others
References
Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY (2006) Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 169(2):566–583. doi:10.2353/ajpath.2006.051066
Bisicchia E, Latini L, Cavallucci V, Sasso V, Nicolin V, Molinari M, D’Amelio M, Viscomi MT (2016) Autophagy inhibition favors survival of rubrospinal neurons after spinal cord hemisection. Mol Neurobiol. doi:10.1007/s12035-016-0031-z
Bursch W, Ellinger A, Kienzl H, Torok L, Pandey S, Sikorska M, Walker R, Hermann RS (1996) Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis 17(8):1595–1607
Chen HC, Fong TH, Lee AW, Chiu WT (2012) Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine 37(6):470–475. doi:10.1097/BRS.0b013e318221e859
Chen B, Yue R, Yang Y, Zeng H, Chang W, Gao N, Yuan X, Zhang W, Shan L (2015) Protective effects of (E)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeine against hydrogen peroxide-induced injury in PC12 cells. Neurochem Res 40(3):531–541. doi:10.1007/s11064-014-1498-5
Chen W, Guo Y, Yang W, Zheng P, Zeng J, Tong W (2016) Involvement of Connexin40 in the protective effects of Ginsenoside Rb1 against traumatic brain injury. Cell Mol Neurobiol 36(7):1057–1065. doi:10.1007/s10571-015-0299-y
Cheong CU, Yeh CS, Hsieh YW, Lee YR, Lin MY, Chen CY, Lee CH (2016) Protective effects of costunolide against Hydrogen Peroxide-Induced injury in PC12 cells. Molecules 21(7):898. doi:10.3390/molecules21070898
Cho IH (2012) Effects of panax ginseng in neurodegenerative diseases. J Ginseng Res 36(4):342–353. doi:10.5142/jgr.2012.36.4.342
Diskin T, Tal-Or P, Erlich S, Mizrachy L, Alexandrovich A, Shohami E, Pinkas-Kramarski R (2005) Closed head injury induces upregulation of Beclin 1 at the cortical site of injury. J Neurotrauma 22(7):750–762. doi:10.1089/neu.2005.22.750
Erlich S, Shohami E, Pinkas-Kramarski R (2006) Neurodegeneration induces upregulation of Beclin 1. Autophagy 2(1):49–51
Goldshmit Y, Kanner S, Zacs M, Frisca F, Pinto AR, Currie PD, Pinkas-Kramarski R (2015) Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci 68:82–91. doi:10.1016/j.mcn.2015.04.006
Guo Z, Cao G, Yang H, Zhou H, Li L, Cao Z, Yu B, Kou J (2014) A combination of four active compounds alleviates cerebral ischemia-reperfusion injury in correlation with inhibition of autophagy and modulation of AMPK/mTOR and JNK pathways. J Neurosci Res 92(10):1295–1306. doi:10.1002/jnr.23400
Huang F, Li YN, Yin F, Wu YT, Zhao DX, Li Y, Zhang YF, Zhu QS (2015) Ginsenoside Rb1 inhibits neuronal apoptosis and damage, enhances spinal aquaporin 4 expression and improves neurological deficits in rats with spinal cord ischemia-reperfusion injury. Mol Med Rep 11(5):3565–3572. doi:10.3892/mmr.2015.3162
Isaac L, Pejic L (1995) Secondary mechanisms of spinal cord injury. Surg Neurol 43(5):484–485
Jang M, Lee MJ, Choi JH, Kim EJ, Nah SY, Kim HJ, Lee S, Lee SW, Kim YO, Cho IH (2016) Ginsenoside Rb1 attenuates acute inflammatory nociception by inhibition of neuronal ERK phosphorylation by regulation of the Nrf2 and NF-kappaB Pathways. J Pain 17(3):282–297. doi:10.1016/j.jpain.2015.10.007
Jensen EC (2013) Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec 296(3):378–381. doi:10.1002/ar.22641
Kanno H, Ozawa H, Sekiguchi A, Itoi E (2009a) The role of autophagy in spinal cord injury. Autophagy 5(3):390–392
Kanno H, Ozawa H, Sekiguchi A, Itoi E (2009b) Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis 33(2):143–148. doi:10.1016/j.nbd.2008.09.009
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E (2011) Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine 36(22):E1427–E1434. doi:10.1097/BRS.0b013e3182028c3a
Kim SO, You JM, Yun SJ, Son MS, Nam KN, Hong JW, Kim SY, Choi SY, Lee EH (2010) Ginsenoside rb1 and rg3 attenuate glucocorticoid-induced neurotoxicity. Cell Mol Neurobiol 30(6):857–862. doi:10.1007/s10571-010-9513-0
Kim HJ, Kim P, Shin CY (2013) A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res 37(1):8–29. doi:10.5142/jgr.2013.37.8
Lee MS, Yang EJ, Kim JI, Ernst E (2009) Ginseng for cognitive function in Alzheimer’s disease: a systematic review. J Alzheimer’s Dis 18(2):339–344. doi:10.3233/JAD-2009-1149
Lee MJ, Jang M, Choi J, Chang BS, Kim SH, Kwak YS, Oh S, Lee JH, Chang BJ, Nah SY, Cho IH (2016) Korean red Ginseng and Ginsenoside-Rb1/-Rg1 alleviate experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 Cells and upregulating regulatory T cells. Mol Neurobiol 53(3):1977–2002. doi:10.1007/s12035-015-9131-4
Lepine S, Allegood JC, Edmonds Y, Milstien S, Spiegel S (2011) Autophagy induced by deficiency of sphingosine-1-phosphate phosphohydrolase 1 is switched to apoptosis by calpain-mediated autophagy-related gene 5 (Atg5) cleavage. J Biol Chem 286(52):44380–44390. doi:10.1074/jbc.M111.257519
Liao B, Newmark H, Zhou R (2002) Neuroprotective effects of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in vitro. Exp Neurol 173(2):224–234. doi:10.1006/exnr.2001.7841
Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda N, Peng H, Aburaya J, Ishihara K, Sakanaka M (1997) Protection of ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci Res 28(3):191–200
Lin CW, Chen B, Huang KL, Dai YS, Teng HL (2016) Inhibition of autophagy by estradiol promotes locomotor recovery after spinal cord injury in rats. Neurosci Bull 32(2):137–144. doi:10.1007/s12264-016-0017-x
Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu J, Ge P, Liang J (2014) Inhibition of autophagy via activation of PI3 K/Akt pathway contributes to the protection of ginsenoside Rb1 against neuronal death caused by ischemic insults. Int J Mol Sci 15(9):15426–15442. doi:10.3390/ijms150915426
Malagelada C, Ryu EJ, Biswas SC, Jackson-Lewis V, Greene LA (2006) RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. J Neurosci 26(39):9996–10005. doi:10.1523/JNEUROSCI.3292-06.2006
Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, Vatner SF, Sadoshima J (2008) Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy 4(4):409–415
Park Y, Liu C, Luo T, Dietrich WD, Bramlett H, Hu B (2015) Chaperone-Mediated Autophagy after Traumatic Brain Injury. J Neurotrauma 32(19):1449–1457. doi:10.1089/neu.2014.3694
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122(6):927–939. doi:10.1016/j.cell.2005.07.002
Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C (2007) Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem 102(4):1242–1255. doi:10.1111/j.1471-4159.2007.04671.x
Radoshevich L, Murrow L, Chen N, Fernandez E, Roy S, Fung C, Debnath J (2010) ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell 142(4):590–600. doi:10.1016/j.cell.2010.07.018
Rivlin AS, Tator CH (1977) Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg 47(4):577–581. doi:10.3171/jns.1977.47.4.0577
Rivlin AS, Tator CH (1978) Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol 10(1):38–43
Seo JY, Kim YH, Kim JW, Kim SI, Ha KY (2015) Effects of therapeutic hypothermia on apoptosis and autophagy after spinal cord injury in rats. Spine 40(12):883–890. doi:10.1097/BRS.0000000000000845
Silva NA, Sousa N, Reis RL, Salgado AJ (2014) From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 114:25–57. doi:10.1016/j.pneurobio.2013.11.002
Sirbulescu RF, Zupanc GK (2010) Inhibition of caspase-3-mediated apoptosis improves spinal cord repair in a regeneration-competent vertebrate system. Neuroscience 171(2):599–612. doi:10.1016/j.neuroscience.2010.09.002
Suzuki C, Isaka Y, Takabatake Y, Tanaka H, Koike M, Shibata M, Uchiyama Y, Takahara S, Imai E (2008) Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun 368(1):100–106. doi:10.1016/j.bbrc.2008.01.059
Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, He C, Du H, Zhang L (2014) Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol 49(1):276–287. doi:10.1007/s12035-013-8518-3
Wang Z, Li M, Wu WK, Tan HM, Geng DF (2008) Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther 22(6):443–452. doi:10.1007/s10557-008-6129-4
Xue JF, Liu ZJ, Hu JF, Chen H, Zhang JT, Chen NH (2006) Ginsenoside Rb1 promotes neurotransmitter release by modulating phosphorylation of synapsins through a cAMP-dependent protein kinase pathway. Brain Res 1106(1):91–98. doi:10.1016/j.brainres.2006.05.106
Yang H, Cheng XP, Li JW, Yao Q, Ju G (2009) De-differentiation response of cultured astrocytes to injury induced by scratch or conditioned culture medium of scratch-insulted astrocytes. Cell Mol Neurobiol 29(4):455–473. doi:10.1007/s10571-008-9337-3
Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J, Lin BB, Zhu SP, Lin L, Gan CS, Fu XB, Li XK, Xu HZ, Xiao J (2013) Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol 48(3):452–464. doi:10.1007/s12035-013-8432-8
Zhou C, Zhong W, Zhou J, Sheng F, Fang Z, Wei Y, Chen Y, Deng X, Xia B, Lin J (2012) Monitoring autophagic flux by an improved tandem fluorescent-tagged LC3 (mTagRFP-mWasabi-LC3) reveals that high-dose rapamycin impairs autophagic flux in cancer cells. Autophagy 8(8):1215–1226. doi:10.4161/auto.20284
Zhou K, Sansur CA, Xu H, Jia X (2017) The temporal pattern, flux, and function of autophagy in spinal cord injury. Int J Mol Sci 18(2):466. doi:10.3390/ijms18020466
Acknowledgements
This work was supported by National Natural Science Foundation of China (81571190, 81371350, 31671071), and Foundation of Zhejiang Educational Committee (Y201636785).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Human and Animal Rights
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
10571_2017_527_MOESM1_ESM.tif
Supplementary material 1 (TIFF 1542 kb) Supplementary Fig. 1 Rb1 downregulates autophagy in PC12 cells after scratch injury. a Representative phase-contrast microscopic images of scratch-wound PC12 cells at 6, 12, and 24 h. Scale bars are 50 μm. c The wound closure rate of scratch-wound PC12 cells at 6, 12, and 24 h. b Immunofluorescent staining of LC3 (Green), while the nuclei are labeled with DAPI (blue). Scale bars are 10 μm. d Fluorescence intensity of LC3 signal. Data are mean ± SD, n = 3 per group. * P < 0.05 versus control group. “※” represents the scratch injury region
Rights and permissions
About this article
Cite this article
Wang, P., Lin, C., Wu, S. et al. Inhibition of Autophagy is Involved in the Protective Effects of Ginsenoside Rb1 on Spinal Cord Injury. Cell Mol Neurobiol 38, 679–690 (2018). https://doi.org/10.1007/s10571-017-0527-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-017-0527-8