Abstract
Background and Objectives
No treatment modalities have been identified to prevent neuron damage induced by traumatic brain injury (TBI). The objective of this study was to investigate whether ginsenoside Rb1 (GS-Rb1) could be utilized to exert neuroprotective effects in TBI.
Methods
Lateral fluid percussion injury (LFPI) was used to induce an experimental TBI model. Lewis rats were divided into a GS-Rb1 group (5, 10, 20 mg/kg, intraperitoneally injected daily), a sham group, and a vehicle group. Neurological impairments were assessed with brain water content, Evans blue extravasation, neurological deficit scores, and Morris water maze test. TUNEL and NeuN staining were utilized to detect neuron apoptosis. The relative expression of apoptosis- and autophagy-relevant molecules were assayed with real-time PCR and western blot.
Results
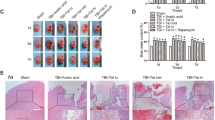
GS-Rb1 inhibited TBI-induced brain edema and Evans blue extravasation in a dose-dependent manner. Furthermore, GS-Rb1 improved neurological impairments with diminished neurological deficit scores, decreased escape latencies, increased time in the target quadrant, and increased number of platform site crossings. GS-Rb1 protected against neuron apoptosis with downregulated Bax expression and upregulated Bcl-2 expression. It was worth noting that TBI increased the LC3II/LC3I ratio and upregulated the relative expression of Beclin-1, Atg-7, and Atg-3; moreover, TBI downregulated the relative expression of P62. The administration of GS-Rb1 further strengthened the relative expression of autophagy-related molecules.
Conclusions
GS-Rb1 alleviates neurological impairments induced by TBI with upregulated autophagy.






Similar content being viewed by others
References
Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. 2017;26(7):1118–30.
Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104(2):213–38.
Kompanje EJO, van Dijck J, Chalos V, van den Berg SA, Janssen PM, Nederkoorn PJ, van der Jagt M, Citerio G, Stocchetti N, Dippel DWJ, et al. Informed consent procedures for emergency interventional research in patients with traumatic brain injury and ischaemic stroke. Lancet Neurol. 2020;19(12):1033–42.
Jiang JY, Gao GY, Feng JF, Mao Q, Chen LG, Yang XF, Liu JF, Wang YH, Qiu BH, Huang XJ. Traumatic brain injury in China. Lancet Neurol. 2019;18(3):286–95.
Jinadasa S, Boone MD. Controversies in the management of traumatic brain injury. Anesthesiol Clin. 2016;34(3):557–75.
Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17(11):647–61.
Chang C, Jensen LE, Hurley JH. Autophagosome biogenesis comes out of the black box. Nat Cell Biol. 2021;23(5):450–6.
Liu CL, Chen S, Dietrich D, Hu BR. Changes in autophagy after traumatic brain injury. J Cereb Blood Flow Metab. 2008;28(4):674–83.
Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54(3):437–53.
Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383(16):1564–76.
Zhou P, Xie W, Luo Y, Lu S, Dai Z, Wang R, Zhang X, Li G, Sun G, Sun X. Inhibitory effects of ginsenoside Rb1 on early atherosclerosis in ApoE−/− mice via inhibition of apoptosis and enhancing autophagy. 2018;23(11):2912.
Zhang Z, Li D, Xu L, Li HP. Sirt1 improves functional recovery by regulating autophagy of astrocyte and neuron after brain injury. Brain Res Bull. 2019;150:42–9.
Cui CM, Gao JL, Cui Y, Sun LQ, Wang YC, Wang KJ, Li R, Tian YX, Cui JZ. Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. Mol Med Rep. 2015;12(2):2323–8.
Rajput SA, Shaukat A, Rajput IR, Kamboh AA, Iqbal Z, Saeed M, Akhtar RW, Shah SAH, Raza MA, El Askary A, et al. Ginsenoside Rb1 prevents deoxynivalenol-induced immune injury via alleviating oxidative stress and apoptosis in mice. Ecotoxicol Environ Saf. 2021;220: 112333.
Li DW, Zhou FZ, Sun XC, Li SC, Yang JB, Sun HH, Wang AH. Ginsenoside Rb1 protects dopaminergic neurons from inflammatory injury induced by intranigral lipopolysaccharide injection. Neural Regen Res. 2019;14(10):1814–22.
Chen X, Huang T, Zhang J, Song J, Chen L, Zhu Y. Involvement of calpain and p25 of CDK5 pathway in ginsenoside Rb1’s attenuation of beta-amyloid peptide25–35-induced tau hyperphosphorylation in cortical neurons. Brain Res. 2008;1200:99–106.
Ardah MT, Paleologou KE, Lv G, Menon SA, Abul Khair SB, Lu JH, Safieh-Garabedian B, Al-Hayani AA, Eliezer D, Li M, et al. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol Dis. 2015;74:89–101.
Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu J, Ge P, Liang J. Inhibition of autophagy via activation of PI3K/Akt pathway contributes to the protection of ginsenoside Rb1 against neuronal death caused by ischemic insults. Int J Mol Sci. 2014;15(9):15426–42.
Chen W, Guo Y, Yang W, Zheng P, Zeng J, Tong W. Involvement of Connexin40 in the protective effects of ginsenoside Rb1 against traumatic brain injury. Cell Mol Neurobiol. 2016;36(7):1057–65.
Correll EA, Ramser BJ, Knott MV, McCullumsmith RE, McGuire JL, Ngwenya LB. Deficits in pattern separation and dentate gyrus proliferation after rodent lateral fluid percussion injury. IBRO Neurosci Rep. 2021;10:31–41.
Chen W, Guo Y, Yang W, Zheng P, Zeng J, Tong W. Protective effect of ginsenoside Rb1 on integrity of blood–brain barrier following cerebral ischemia. Exp Brain Res. 2015;233(10):2823–31.
Hatashita S, Hoff JT, Salamat SM. Ischemic brain edema and the osmotic gradient between blood and brain. J Cereb Blood Flow Metab. 1988;8(4):552–9.
Zeng X-J, Li P, Ning Y-L, Zhao Y, Peng Y, Yang N, Zhao Z-A, Chen J-F, Zhou Y-G. Impaired autophagic flux is associated with the severity of trauma and the role of A2AR in brain cells after traumatic brain injury. Cell Death Dis. 2018;9(2):252.
Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021(1):41–53.
Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett. 2011;487(1):70–2.
Zhu J, Jiang Y, Wu L, Lu T, Xu G, Liu X. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience. 2012;202:342–51.
Gao XQ, Yang CX, Chen GJ, Wang GY, Chen B, Tan SK, Liu J, Yuan QL. Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J Ethnopharmacol. 2010;132(2):393–9.
Kim KA, Jung IH, Park SH, Ahn YT, Huh CS, Kim DH. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS ONE. 2013;8(4): e62409.
Dai SN, Hou AJ, Zhao SM, Chen XM, Huang HT, Chen BH, Kong HL. Ginsenoside Rb1 ameliorates autophagy of hypoxia cardiomyocytes from neonatal rats via AMP-activated protein kinase pathway. Chin J Integr Med. 2019;25(7):521–8.
Liu X, Chen J, Sun N, Li N, Zhang Z, Zheng T, Li Z. Ginsenoside Rb1 ameliorates autophagy via the AMPK/mTOR pathway in renal tubular epithelial cells in vitro and in vivo. Int J Biol Macromol. 2020;163:996–1009.
Qiao L, Zhang X, Liu M, Liu X, Dong M, Cheng J, Zhang X, Zhai C, Song Y, Lu H, et al. Ginsenoside Rb1 enhances atherosclerotic plaque stability by improving autophagy and lipid metabolism in macrophage foam cells. Front Pharmacol. 2017;8:727.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The study was supported by the Key R & D project of Jiangxi Provincial Department of Science and Technology (20203BBGl73172); the scientific research project of Jiangxi Provincial Department of Education (GJJ200165, GJJ190022); the key youth project of Jiangxi Natural Science Foundation (20202ACBL216005); the National Natural Science Foundation of China (NSFC: 82171366, 81960236).
Conflict of interest
The authors declare that they had no conflicts of interest.
Ethics approval
The study protocol was approved by the Animal Ethics Committee of the First Affiliated Hospital of Nanchang University. All institutional guidelines for the care of the laboratory animals were followed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data could be obtained upon reasonable request to the corresponding authors.
Code availability
Not applicable.
Author contributions
S.Z., W.C., H.D., Y.Q., Z.W., J.F., D.R., J.D., and B.J. conducted the experiments, and collected and analyzed the data; S.Z., W.C., J.D., B.J., and J.F. conceived the study; S.Z., W.C., H.D., Y.Q., Z.W., J.F., D.R., J.D., B.J., and J.F. wrote the original manuscript and revised the paper.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, S., Chen, W., Ding, H. et al. Involvement of Autophagy in the Protective Effects of Ginsenoside Rb1 in a Rat Model of Traumatic Brain Injury. Eur J Drug Metab Pharmacokinet 47, 869–877 (2022). https://doi.org/10.1007/s13318-022-00799-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00799-0