Abstract
Vanillin is an active ingredient found in the crop ‘vanilla’ and is traditionally extracted from the ‘vanilla pod’. Vanillin intrinsically is not a suitable candidate for imparting durable functional features into textile substate due to its smaller chemical structure which leads to leaching of the same during washing operation. To enlarge the structure, in the present study, vanillin has been converted into 4-(benzylamino) methyl))-2-methoxyphenol vanillin derivative (reduced Schiff base) with considerable amount of yield by using a simple one-step process and the synthesized product has been characterized by 1H, C13 NMR, FTIR, and Raman analysis. Thereafter, the reduced Schiff base of vanillin (RSB) has been integrated on cotton as well as polyethylene terephthalate (PET) fabric using high temperature high pressure (HT-HP) technique for imparting multiple functionalities. FESEM EDX analysis has confirmed the integration of RSB on both the fabrics by revealing uniform presence of the nitrogen (of the synthesized derivative) on the treated textile materials. Both types of functionalized textiles have demonstrated appealing color shades with an excellent antimicrobial activity of about 90% against Escherichia coli (E. coli) bacteria. The treated fabrics could cater pleasing fragrance and exhibit 90% antioxidant properties. Moreover, enlarged vanillin derivative in the form of RSB can retain its properties in the fabrics even after repeated machine launderings. RSB-treated cotton fabric has shown ultra-violet protection factor (UPF) of 38 which drops to 24 after washing whereas in case of PET treated fabric, the observed UPF values are 265 and 164 before and after washing, respectively. The RSB treatment has been found to be cytotoxically secure and biocompatible as tested on the PET fabric. Other required properties of the treated fabrics such as water absorbency, flexibility, etc. have also been found to be intact. Thus, the presented study reveals a new class of safe material that can be derived from eco-friendly vanillin and has the potential to replace hazardous chemicals that are currently used in textile chemical processing industries.
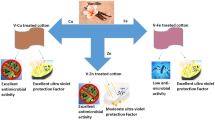
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cotton and polyester fibers cover a substantial part in textile manufacturing due to their exceptional properties. Cotton is a natural fibre having manifold lucrative properties such as comfort, softness, and so on, and thus is a fibre of choice for clothing. Cotton is a crop that is cultivated throughout the world, whereas polyethylene terephthalate (PET) is a synthetic polyester group fibre. In the last decade, the demand for PET fibre has increased exponentially. The polyester market has grown very sharply and is accounted for 45% of the total market (Textile 2010). Regrettably, cotton fabric has issues with bacterial growth as well as a low ultra-violet protection factor (UPF), whereas PET has major issues with bacterial adhesion due to its hydrophobic characteristic. However, in today's world, people are more concerned about their health and hygiene, and thus there is a growing demand for textile substrates with functional properties which stimulated the research community to develop fabrics with multifunctional properties such as antimicrobial, antioxidant as well as harmful UV ray protection. Various finishing agents (natural as well as synthetic) are applied to textile substrates to impart these functionalities. Finishing agents commonly used in the textile industry are often unfriendly to the environment. On the other hand, natural occurring finishing agents have issues with integration over textiles as well as wash durability. Vanillin (p-vanillin) has not been closely examined and explored in the textile field. It has only been investigated as a carrier in polyester dyeing (Pasquet et al. 2013). Aside from its function as a carrier, its finishing efficacy as an antibacterial agent is not very satisfactory due to its small structure and topological surface area (TPSA) (Gressier et al. 2019). Among the isomers (ortho and para), p-vanillin is considerably safer and has been explored in various industries such as pharmaceuticals, food and beverages, and fragrance, but has not thoroughly been investigated in the textile segment. Traditionally, two or more compounds have been applied to textiles to achieve multifunctional properties, which are associated with various issues such as compatibility, multiple process sequences to integrate them into textiles, and so on. These multi-step processes consume a lot of water and energy, and they invite a variety of other problems associated with toxic effluent which make the entire process unsustainable. As a result, there is a growing demand for a single finishing agent integrated into a textile to impart effective multifunctional properties. Natural materials have recently gained prominence and interest in this concept. However, the application of natural materials on textile substrates remains challenging to the need for a higher add-on percentage due to the presence of multiple non-essential components as well as less-reactive sites in their chemical structure. On the other hand, the mechanism of interaction of finishing agent in cotton and PET fabric is entirely different. Finishing agents interact with amorphous region in cotton fibre and remain there due to various physical interactions such as hydrogen bonds, van der Waals interaction or by forming covalent bonds. Other approach of integration of finishing agent in cotton substrate is physical entrapment of the agent that is soluble at higher temperature during its integration process but insoluble at room temperature during usages. Such an observation has recently been reported (Sharma et al. 2022a), in which the material got entrapped in the cotton fabric when the temperature was reduced, and retained inside in an insoluble form, along with other interactions such as hydrogen bonds, etc. In case of PET, diffusion process of finishing agents in aqueous media is slower due to its highly compact structure with hydrophobic nature. For such fabrics, finishing agents can get mechanically entrapped inside the fabric during the treatment process at above Tg (glass transition temperature) and get retained inside the fabric once the temperature is lowered below Tg as reported elsewhere (Sharma et al. 2022b). Also, the finishing agents or dyes commonly used on PET fabric stimulate a wide range of environmental issues. Cotton, flax, and other cellulosic derivatives have extensively been researched in the last 5 years to achieve long-lasting and very innovative functional applications such as UV protective fabric, fluorescent clothing, contaminant removal, and antibacterial textiles (Mohajerani et al. 2019; Ahmed et al. 2021; Emam et al. 2021a, b, 2022a, b; Marae et al. 2021). Extensive research is also being conducted to develop functional textiles that could be used in military, hospital, and waste-water treatment, among other applications.
Moreover, synthetically prepared materials have the advantage of requiring a low add-on percentage to imbue functionalities into textiles or other substrates, which is even more advantageous when the materials are converted to nano-form (Sharma et al. 2016, 2018; Chawla et al. 2017; Durrani et al. 2020; Rajpoot et al. 2021; Ali and Sharma 2022). However, in many cases, these are proven to be health hazardous or environmentally not a benign solution. As a result, the scientific community is constantly working to replace hazardous chemicals with environmentally friendly alternatives (Pasquet et al. 2013; Basak and Wazed Ali 2018; Shukla et al. 2019). Various bio-based finishing agents, such as lignin-based compounds, pomegranate rind extract, aloe vera gel, and other natural ingredients, are being investigated in this context to impart various functional properties to textile substrates (Ali et al. 2014; Basak and Wazed Ali 2018; Shukla et al. 2019; Joshi et al. 2007). However, in order to achieve satisfactory functionalities, these finishing agents require a high add-on percentage, which stiffens the textile substrates and impairs other useful textile properties.
Vanillin is extracted naturally from vanilla pods and can also be obtained synthetically as well as through biotechnological routes. Vanillin has been used extensively as flavoring substance in various food making industries, perfumery and pharmaceutical industries and it is also potential inhibitor of COVID-19 virus as reported in a recent study (Sinha et al. 2009; Bezerra et al. 2016, 2020; Gallage and Møller 2017; Arya et al. 2021). Because of its inherent structural constituents with various functional groups, vanillin has many beneficial properties such as antioxidant, antimicrobial, anti-inflammatory, anticancer, antifungal, anti sickle cell anemia, and so on. (Ma et al. 2020; Venkata et al. 2020, 2021; Abraham et al. 1991; Tai et al. 2011; Hannemann et al. 2014; Wu et al. 2020). However, its use in the textile industry is limited due to its smaller structural feature, and it easily comes out of the textile structure during washing. Furthermore, in its parent form, its chemical structure prevents it from forming a covalent bond with any of the textile substrates. Recently, researchers have experimentally transformed vanillin into Schiff base to enhance its structural conformation for suitable entrapment into the amorphous region of PET fabric, and it has been possible to generate desirable functional properties with reasonable wash durability (Sharma and Ali 2022b). Although the authors have observed some extent of functional properties on fabric such as antibacterial activity, good UPF value, etc., but the effect against a range of bacteria as well as the antioxidant property are not up to par. Antimicrobial and antioxidant activity are attributed to the presence of functional –OH groups in vanillin structure. The overall structural feature protects the treated fabric from ultraviolet rays.
In the present research work, a unique derivative of vanillin (i.e. reduced Schiff base) with high yield percentage has been synthesized. Initially, vanillin Schiff base has been synthesized in-situ using vanillin and benzyl amine, and afterwards it has been reduced to form reduced Schiff base (RSB) in a simple and cost-effective single step process. The presence of an –NH group in RSB significantly enhances the antioxidant activity as well as antimicrobial efficacy. The single-step preparation with simple reagents is quite useful for commercialization, and the process is, of course, industrially viable. The synthesized RSB has then been integrated into both the cotton and polyester (PET) fabric using a high temperature high pressure (HT-HP) dyeing machine to impart multi-functionalities. The treated textiles have demonstrated significant improvements in properties as compared to the previous study with only Schiff base product (Sharma and Ali 2022b). The present context demonstrates that the synthesized novel RSB molecules can provide multiple functionalities (antimicrobial, antioxidant, UPF, fragrance) on both the cotton and PET textiles, without interfering with other useful physical properties (tensile, absorbance, bending length, etc.) of the fabrics. The functionalized fabrics have a lot of potential for indoor (curtains, hospital bed sheets, etc.) as well as outdoor (tents, stealth, etc.) applications. The same value-added fabric has enormous potential to be explored in reusable mask (usable up to 30 washes) in pandemic situation like Covid-19 also.
Materials and methods
Ready for dyeing (RFD) bleached cotton fabric (40 × 40/132 × 72 twill weave) of 100 g/cm2 (GSM) and PET (plain weave) fabric of 100 g/cm2 (GSM) having no optical brightening agent added into them were purchased from a local market in New Delhi. Vanillin, benzyl amine, 2,2-diphenyl-1-picrylhydrazyl (DPPH), sodium borohydride, methanol, and ethanol were purchased from Central Drug House (CDH) and used in the study without further purification. Analytical grade TLC Silica gel 60 F254 plate and extra pure Agar–Agar were purchased from Merck and Luria broth was purchased from Himedia, respectively which were used in the antibacterial study. CDCl3, Deuterated DMSO and KBr of analytical grade were purchased from Sigma Aldrich and used for NMR and FTIR sample preparation, respectively.
Synthesis of reduced schiff base (RSB)
0.1 mol of vanillin and 0.1 mol of benzyl amine were added at 35 °C in a round bottom flask having three times methanol to that of vanillin. Yellow colored solution was instantaneously formed as both the reagents were mixed. The reaction mass was stirred for 30 min at 35 °C. Afterwards the temperature was lowered down to 15 °C and sodium borohydride (0.1 mol) was added in 3–4 lots. After this addition, the reaction mixture was again stirred for 45 min at 35 °C. TLC was used to check the extent of the reaction for completion (S1 in Appendix). Just after that, methanol was removed using an evaporator, and water was added 5 times with a few drops of dilute hydrochloric acid to keep the pH between 6.5 and 7. The reaction mass was stirred for an additional hour before being filtered under vacuum. The filtered material was dried in a hot air oven at 40 °C until it reached a constant weight. The reaction was replicated three times, and the same observation was obtained with nearly the same yield each time. The reaction scheme for the synthesis of RSB is depicted in Fig. 1 with the advantage of using a simple reagent without heavy metals which is different than the previously reported process (Scipioni et al. 2018).
Characterization of the synthesized RSB
Thin layer chromatography to monitor the extent of reaction
Desirable dimensions of TLC Silica gel 60 F254 plates (UV active plates) were cut and used to study the progress of the reaction after complete addition of the reagents.
Nuclear magnetic resonance study for structural analysis
1H and 13C NMR spectra of the synthesized RSB and vanillin were captured using Bruker Machine of 400 MHz. In an NMR tube, 5 mg of sample was dissolved in deuterated solvent and placed in an NMR instrument.
FTIR analysis to investigate the presence of functional groups
FTIR analyzer (model Nicolet iS50FT-IR of Thermo Scientific company) was used to investigate the functional groups present in the materials. The samples were scanned from 400 to 4500 cm−1 and data was collected as needed.
Raman analysis to reaffirm the functional groups
Raman analyzer (Reni Shaw model) was used to examine the functional groups of the material. Both the vanillin and the prepared RSB were subjected to Raman analysis. Samples were exposed at 785 nm laser light to analyze them in the 200–2500 cm−1 wavenumber range.
X-ray diffraction to investigate the micro-structure the of materials
The X'pert pro machine was used to investigate the extent of crystallinity of the synthesized samples at a scan rate of 0.02 s/step in the 2θ range from 10° to 80°. The sample was exposed to X-ray (Cu-Kα radiation) of wavelength 1.54 Å and data was recorded accordingly.
Pretreatment of cotton and PET fabric
1 g/L of non-ionic soap (lissapol-N) in a material-to-liquor ratio of 1:20 was used to pretreat cotton and PET fabric separately at 70 °C for 30 min. The fabric was then washed twice with water to remove any finishing agent that had remained on the surface.
Incorporation of synthesized RSB into cotton and PET fabric
1 g of each fabric (cotton and PET) was cut and placed in two separate infrared (IR) beakers. Fabric (material or M) to water (Liquor or L) in the ratio of 1:30 (M: L) was maintained in each beaker containing 100 mg RSB separately.
Beakers were then placed in an IR dyeing machine and heated at 120 °C for 20 min. Once the treatment was over, the temperature of the beakers was reduced to 40 °C, and the treated fabrics were removed out of the beakers. The surface adhered material was thoroughly washed with fresh tap water. Finally, the treated fabrics were washed with 1 g/L non-ionic detergent, followed by regular water, and air dried.
Color strength of the treated cotton and PET fabric
Premier Color Scan Spectrophotometer (Model no 5100, Lambda (35 model)) equipped with an integrating sphere was used to measure the color value of treated fabrics. Color parameters were set according to the standard method (Basak and Wazed Ali 2018).
SEM EDX to analyze the elemental presence on the treated fabric
SEM was used to scan RSB-treated fabrics, and EDX analysis was performed to determine the presence of elements. The treated samples were examined using a TM3000 tabletop microscope (HITACHI, Swift ED3000). Prior to scanning, fabric samples were coated with a thin conducting layer of Gold/Palladium of nearly 100 Å thickness using a 20 kV accelerating voltage to make the samples conductive in nature.
Assessment of antimicrobial activity of the treated fabric
Standard AATCC 100 method was used to analyze the antibacterial activity of the RSB treated fabrics. Colony counter was used to calculate the number of colony forming unit (cfu) for the control and the treated fabrics. The percentage in bacteria colony reduction (BCR) was calculated by using the following formula:
where, A is the number of viable microorganisms for control sample and B is the number of viable microorganisms in presence of treated fabric sample.
Antioxidant activity of the treated fabric
The antioxidant activity of the RSB treated fabrics was measured using standard 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical entrapment method. DPPH in methanol solution depicts absorption maxima at 517 nm. Untreated and treated fabric samples of the same dimension were placed separately in a known concentration of DPPH solution for 30 min in the dark, and absorbance was measured. The decrease in absorbance of DPPH solution at 517 nm in the presence of fabric is directly proportional to the fabric's antioxidant activity. A UV–visible spectrophotometer (Model UV 2450, Shimadzu) was used to measure the absorbance.
Assessment of ultra-violet protection factor (UPF)
The ultra-violet protection factor (UPF) of untreated and RSB-treated fabric samples was determined using a UPF tester (Model UPF 2000, Labsphere). The UPF of the samples was determined using the standard method (AATCC 183:2014). Each sample was scanned three times in different locations and the average values are reported in the text.
Assessment of tensile strength
Tensile strength of the untreated and treated textiles was measured by standard tensile testing method (ASTM D5034, grab test method) using Tinius Olsen tensile testing machine (Model: H5KS). Sample of 20 cm × 5 cm dimension was cut and the tensile strength was measured at a speed of 300 mm/min.
Washing fastness test
Washing fastness of the treated fabrics was tested using a laboratory grade launder-o-meter (R.B Electronic and Engineering) and by following the standard AATCC 61 1A method. During the washing process, ECE phosphate reference detergent was used. Fabric sample of dimension 15 cm × 5 cm was cut and treated in 200 mL water containing standard recipe (AATCC 61A) at 40 °C for 45 min.
In vitro cytotoxicity assay
To evaluate in vitro cytotoxicity of RSB treated sample, an MTT (MTT is 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay that is utilized for studying cell toxicity) reduction colorimetric assay was used on a L929-Mouse connective tissue cell line, following ISO 10993-5. Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum was used to culture all the cells. Primarily, 1 × 104 cells were developed in each well of a 96 well plate at 37 °C and 5% CO2 for 24 h. Then, for another 24 h, the cells were treated with 1 g of fabric sample. The MTT solution (5 mg/mL in PBS) was added to it after the treatment. The cells were incubated at 37 °C for 3 h in a cell culture incubator. Dimethyl sulfoxide was used to dissolve the formazan crystals, and the absorbance (optical density (O.D.)) was recorded at 570 nm in a 96 well plate reader. With the following Eq. (3), the cell viability was calculated:
Results and discussion
A very simple and industrially viable process was used to synthesize RSB. Analytical techniques such as NMR, FTIR, XRD, Raman were used to characterize the synthesized product. Finally, the synthesized RSB was integrated separately into cotton and polyester (PET) fabric to impart multifunctional effects using HT-HP IR dyeing machine.
Material identification by nuclear magnetic resonance study
Nuclear Magnetic Resonance (NMR) technique was employed to confirm the formation of RSB from vanillin (as represented in Fig. 2a, b). Different hydrogen absorbs with different chemical shifts in different environments. For RSB, a well-resolved spectrum was obtained, confirming the formation of the structure. Vanillin represents a sharp aldehyde peak at 9.8 ppm (singlet), which is absent in the RSB spectrum, and two equal peaks at 3.57 ppm (singlet) and 3.65 ppm (singlet) have been observed due to the presence of two –CH2 groups in RSB. A broader peak is found in the spectrum at 8.75 ppm (singlet) due to –OH groups. The presence of a –NH peak at 2.42 ppm (singlet) in RSB confirms the formation of the structure. The –OMe (singlet) peak is present in both vanillin and RSB at nearly 3.8 ppm. Peaks in the spectra of the solvents used for analysis are not identified. CDCl3, deuterated DMSO and water peak are observed at 7.26 ppm, 3.5 ppm and 2.5 ppm, respectively in the Fig. 2a or b). Area under the peak also represents the number of protons present in the chemical structure. Peak splitting due to neighboring hydrogen atoms is also observed. Aromatic peaks due to benzylamine are observed in the region 7.23 ppm (multiplet, 4H) and 7.32 ppm (multiplet, 1H) in both case, vanillin and RSB treated fabric.
13C NMR spectra of vanillin and RSB were recorded to confirm the formation of RSB from vanillin as shown in Fig. 3a, b. The spectrum of vanillin shows that the –CHO peak at 190.95 ppm is present in vanillin but not in RSB. The –C–OH peak can be seen in both the spectra. Vanillin has a peak position of 147.21 ppm, while RSB has a peak position of 145.67 ppm. The peak at 52.56 ppm is observed in the aliphatic region, corresponding to –CH2–CH2. Additional peaks in the aromatic region are observed due to the benzyl amine moiety in RSB, confirming the formation of RSB from vanillin. Position 7 in Fig. 3b represents four carbon atoms (visible in zoom spectrum), whereas two equal peaks represent four carbon atoms.
Functional group analysis of the synthesized material
FTIR study
FTIR study was conducted to confirm the formation of RSB. FTIR spectra of vanillin and the prepared RSB were recorded from 400 to 3500 cm−1 and are represented in Fig. 4a, b. Vanillin absorbs at 1666 cm−1 due to the CHO group, whereas RSB does not absorb at this wavelength (Thirunavukkarasu et al. 2018). A new band at 3288 cm−1 for –NH stretching is observed, confirming the formation of a reduced Schiff base. However, it interferes with the –OH group present, reducing the peak intensity of –OH group. 1H NMR spectrum clearly indicates the absorbance at 8.75 ppm which signifies the presence of –OH group in the structure.
Raman analysis
The functional groups present in vanillin and synthesized RSB were identified using Raman spectra, recorded in 200–2000 cm−1 range as shown in Fig. 5. Vanillin absorbs majorly at 1670, 1590, 1270, 1170, 955, 816, 733, 635, 540 and 430 cm−1. Raman band at 1670 cm−1 corresponds to vanillin aldehyde group (Thirunavukkarasu et al. 2018) (Fig. 5a), whereas in case of reduced Schiff base peak at 1670 cm−1 was absent which clearly indicates formation of RSB from vanillin represented in Fig. 5b.
The band near 1170 cm−1 is caused by C–O stretching of –OCH3. In case of RSB, newly formed bands at 1603 and 1642 cm−1 correspond to –C=C– of the benzene ring.
The formation of RSB is indicated by the formation of a new covalent bond and a change in the end aldehyde group to amine, which is clearly visible in the spectrum (Fig. 5a, b). Furthermore, the NMR spectrum of the synthesized compound, as discussed in an earlier section of this article also, supports the formation of RSB.
XRD study
X-Ray Diffraction (XRD) pattern was studied to evaluate the extent of the crystallinity of the material. Both the vanillin and synthesized RSB material were scanned at a rate of 0.02 s/step in the 2θ range from 10° to 80°. Both vanillin and synthesized RSB are found to be crystalline in nature. Figure 6a, b show XRD peaks for vanillin and RSB, respectively.
Vanillin shows many crystalline peaks (Fig. 6a), but the most intense peak appears at 2θ value 13.07° which is also in full agreement with an earlier reported values (Hasanvand and Rafe 2019). Small peaks are also visible for vanillin at 2θ value 23.8° and 39.8° but their intensities are very low as compared to the main peak. XRD pattern of RSB shows a main peak at 2θ value 20°, which confirms the formation of a new compound and is also supported by NMR results.
Incorporation of RSB into fabrics
HT-HP IR dyeing machine was used to incorporate RSB in both cotton and PET fabrics separately. Vanillin, on the other hand, is unable to retain in fabrics due to its very small structure. As PET is hydrophobic in nature and vanillin has a high topological polar surface area (TPSA) (Gressier et al. 2019), the interaction between PET and vanillin is not much stronger, and vanillin, due to its smaller structural feature, leaches out of fabric after washing, making it unsuitable for use as a finishing agent (Gressier et al. 2019). As previously reported, there are not many strong attractive forces in retaining vanillin within the structure of cotton (only hydrogen bonds and some degree of solubility) (Sharma et al. 2022a) also. In the current study, reduced Schiff base is successfully incorporated into both cotton and PET fabric through good interaction, which could be attributed to two major factors: (1) structure enlargement of vanillin after the formation of RSB and (2) reduced solubility of RSB. However, integration of RSB into PET fabric is done at high temperature (120 °C) wherein solubility of RSB gets increased (due to very high RSB to water ratio, i.e. 1:300) and it penetrates within the amorphous zone of PET structure. Once the temperature gets down at the end of the treatment process, the molecules become insoluble within the structure and do not escape out very easily at room temperature or even in washing conditions [which is lower temperature than Tg (glass transition)] of PET. In case of cotton, structural (chemical) enlargement of vanillin in the form of RSB increases the attraction forces with cellulosic backbones. As the molecules also have hydrophilic characteristics due presence of –OH, –NH group, they penetrate within the cotton structure at higher temperature (because of enhanced solubility) and interact with cellulosic structure via hydrogen bonds. Also, the solubility of RSB gets reduced at room temperature or washing temperature (as mentioned in case of PET also) and these combined effects make the finishing more durable on cotton fabric. The water absorbency of the treated fabric is also not compromised, as determined by a drop absorbency test. Cotton fabrics, both untreated and treated, showed water drop absorbency in less than 5 s (i.e. very good absorbency). Bending length of the untreated and treated samples is measured to determine the flexibility. The flexibility of the treated fabric is found to be almost unaltered because the required add-on percentage was very low (only 1–1.5%). Untreated cotton fabric shows an average bending length of 3.5 cm, but after RSB treatment, the same is measured as 3.6 cm, whereas untreated and treated PET fabrics represent the value of 2.3 cm and 2.35 cm, respectively.
The finished fabrics also show good tensile strength retention (as discussed in the later part of the article). The percentage of RSB on treated cotton and PET was determined by UV–Vis spectrophotometer using Beer-Lambert’s law. RSB solutions with known concentrations were prepared in DMF (dimethylformamide—30 mL in each solution), and an RSB calibration curve was derived from absorption data. The treated cotton and PET fabrics were then dipped into 30 mL DMF and heated at 70 °C for 30 min to allow all incorporated RSB to be leached out of the fabric. The absorbance of this DMF solution was measured with a UV–Vis spectrophotometer, and the corresponding concentration was calculated using the calibration curve. Cotton fabric shows nearly 20 mg RSB content, whereas the same is measured as nearly 45 mg for PET fabric.
Assessment of the presence of RSB in the treated fabrics using color measurement and wash durability study
HT-HP machine with M:L ratio of 1:30 was used to treat 1 g of cotton and PET fabric (each separately) with 100 mg RSB. Both types of treated fabrics show an appealing brownish shade. Figure 7a–c represent control cotton (CC), 100 mg RSB treated cotton (RSBC) and 100 mg RSB treated cotton after 30 home laundry washes (RSBC 6W), respectively. On the other hand, Fig. 7d–f depict control PET (CP), 100 mg RSB treated PET (RSBP) and 100 mg RSB treated PET after 30 home laundry (RSBP 6W). Even after 6 machine washes, there is no discernible change in the colour value (K/S value represents colour value) of the treated cotton and PET fabric. Table 1 shows that the colour strength of treated cotton fabric is 0.36 and that of PET fabric the value is 1.54. As mentioned in the experimental section, the AATCC 61 1A method was used to investigate the wash fastness of the treated samples. The colour strength of the cotton fabric and the PET fabric is remained as 0.236 and 1.39 after 6 machine washes, respectively. It means that the active ingredient (RSB) remains intact within the fabric structure after multiple washes. Each machine wash is equivalent to 5 home launderings (Sharma and Ali 2022a). The treated fabrics can retain their colour even after 30 home launderings, and the wash durability rating is found to be around 3–4 on a grey scale.
L, a, b values and colour strengths of the untreated and treated fabrics (both before and after washed) are represented in Table 1 (at maximum wavelength, 420 nm).
Evaluation of presence of RSB on fabric using FTIR analysis
FTIR analysis confirms the incorporation of RSB into treated textiles as depicted in Fig. 8a, b. However, bands are quite weak due to a very low add on percentage (i.e. 1–1.5% only) and cannot be distinguished from untreated fabric effectively. Nevertheless, in the case of cotton fabric, if we expand the region 1400–1700 cm−1, we can observe an additional band of –C = C– due to the aromatic structure of RSB. In the case of RSB-treated PET, no additional bond is observed, which could be attributed to the presence of an aromatic ring in the PET structure itself, which interferes with related RSB peaks. On the other hand, the colour value of the treated fabric directly confirms the presence of RSB. The same is supported by EDX analysis, which is discussed further in the article.
Assessment of presence of RSB on cotton and PET fabric through SEM–EDX
FESEM EDX analysis affirms that RSB treated cotton and PET textiles have a uniform treatment throughout the structure as shown in Fig. 9 and Fig. 11. A peak at 2.2 keV is observed in case of both the untreated and treated sample which is due to gold coating to make the fabric conductive as well as to dissipate the charging caused by moisture in the fabric. The presence of RSB on cotton and PET fabric even after 6 washing cycles is investigated further using FESEM (as shown in Figs. 10 and 12), which reflects the homogeneity of RSB treatment on fabrics. EDX of both the treated fabrics is taken in three different locations, and nearly equal amounts of elements are detected for each measurement. The presence of nitrogen also confirms that RSB is effectively retained in the fabrics even after 6 wash cycles. The FESEM EDX mapping clearly depicts that RSB is uniformly integrated on the textile substrate and is present on individual fibre as shown in Fig. 9b. The particle size of RSB is nearly 500 nm as shown in Fig. 9d. RSB treated cotton contains elements like carbon, nitrogen and oxygen as represented in Fig. 9c, e, f, respectively, where carbon is represented by orange color, nitrogen by magenta color and nitrogen by green color. The presence of elements is represented in Fig. 9g whereas correspondence percentage is reported in Fig. 9h. EDX results depict 63.2% carbon, 35.6% oxygen, and 1.2% nitrogen on the treated fabric.
FESEM mapping and EDX images of RSB treated cotton: a FESEM image, b mapping of RSB, c carbon elements only presented by yellow color, d FESEM zoomed image, e nitrogen elements only presented by magenta color, f oxygen elements presented by green color, g EDX of sample, h elements present in percentage
FESEM mapping and EDX images of RSB treated cotton after 6 wash: a FESEM image, b mapping of RSB, c carbon elements only presented by yellow color, d zoomed FESEM image, e nitrogen elements only presented by magenta color, f oxygen elements presented by green color, g EDX of sample, h elements present in percentage
Figure 10 shows RSB-treated cotton fabric after six wash cycles. A similar observation is made for the treated cotton even after 6 washes, confirming the effective retention of material inside the cotton structure. Even after washing, EDX reveals 63.4% carbon, 35.4% oxygen, and 1.2% nitrogen on the treated fabric, indicating RSB entrapment within the cotton fabric as shown in Fig. 10h.
However, after washing, the treated fabric contains less RSB, which could be attributed to the removal of surface-deposited RSB particles.
Figure 11 shows FESEM images, elemental mapping, and EDX of RSB-treated PET fabric, and Fig. 12 shows the results after 6 wash cycles. Both the treated PET and cotton fabrics show a similar pattern. As shown in Fig. 11b, FESEM EDX mapping clearly shows that RSB is uniformly integrated on PET substrate. RSB-treated PET contains elements such as carbon, nitrogen, and oxygen, as shown in Fig. 11c, e, 11f, where carbon is represented by orange, nitrogen by magenta, and nitrogen by green colour. Elements are shown in percentage (%) in Fig. 11g, and correspondence percentage values are shown in Fig. 11h. On the treated fabric, EDX analysis revealed 75.8% carbon, 22.5% oxygen, and 1.8% nitrogen.
FESEM mapping and EDX images of RSB treated PET—a FESEM image, b mapping of RSB, c carbon elements only presented by yellow color, d zoomed FESEM image, e nitrogen elements only presented by magenta color, f oxygen elements presented by green colour, g EDX of sample, h elements present in percentage
After 6 washes, RSB treated PET showed 77.4% carbon, 21.6% oxygen, and 1% nitrogen as shown in Fig. 12h, indicating that RSB is retained on fabric very effectively even after repeated washing operations.
Assessment of antimicrobial activity of treated cotton and PET fabrics
The antimicrobial efficacy of untreated and RSB-treated fabrics was assessed using a standard method (AATCC100, colony counting method). RSB-treated fabrics demonstrate excellent antimicrobial activity against the tested E. coli bacteria.
The antimicrobial activity is retained to nearly 85–90% after 30 home launderings, as illustrated in Fig. 13. The antimicrobial activity of the treated fabrics was tested three times, and the results are consistent. As a result, these textiles have the potential to be used as antimicrobial products in the healthcare sector, as well as in a variety of other indoor and outdoor applications.
Assessment of antioxidant activity of treated cotton and PET fabrics
The antioxidative activity of the treated fabrics was investigated using the ‘2, 2-diphenyl-1-picryl-hydrazyl-hydrate’ (DPPH) method. In a UV–Vis spectrophotometer, DPPH free radical solution absorbs at 517 nm, whereas antioxidant textiles or materials absorb DPPH and reduce the intensity of the absorbance peak at 517 nm.
Figure 14 depicts the basic mechanism and moieties responsible for antioxidant properties. The presence of –NH (in addition to –OH) in RSB makes it a strong antioxidant agent, and thus the treated textiles demonstrate very significant antioxidant activity which is more than 90% with respect to control sample (Scipioni et al. 2018). The experiments were repeated three times to eliminate any experimental errors, but the same result (more than 90% antioxidant activity) was obtained for each measurement.
Assessment of UPF of treated cotton and PET fabrics
The treated textiles show an excellent ultra-violet protection in addition to colour value, antimicrobial activity, antioxidant property, and fragrance. However, different fabric parameters such as fabric construction, fabric areal density, chemical treatment, etc. influence the ultra-violet protection factor (UPF) of textiles. Because of its favorable structural configuration, RSB displays a high UPF value on treated textiles in the present study. Despite having a low add-on percentage on the treated textiles, the fabrics show a high UPF rating. After 6 home washing cycles, the UPF value changes marginally. The UPF of treated cotton is 38.66 on average of three readings and decreases to 24 after 30 home washing operations, whereas untreated textile registers UPF of only 9. The UPF value of RSB-incorporated PET fabric is 265 before washing and decreases to 164 after washing, whereas untreated PET exhibits a UPF value of 62. It directly indicates that the active ingredient (RSB) is retained in the PET structure even after 30 home washings. Table 2 summarizes the results of all experiments conducted according to a standard procedure (AATCC 183:2014).
Cytotoxic study of RSB treated PET fabric
The RSB-treated PET sample was tested for cytotoxicity in vitro at various extract concentrations from 10 to 100%. The standard experiment was carried out on the mouse connecting tissue cell line L929. It was found that treated PET fabric is completely safe and biologically compatible in the conc. range 10–100% as represented in Fig. 15, with a visible cell percentage of greater than 70%. If the percentage is less than 70%, it is potentially cytotoxic (Standard method ISO 10993-5:2009 (E)). As a result, it is prudent to conclude that the RSB treatment on textile substrates is harmless and the treated textiles can be used for apparel, various household, and other technical textile applications.
Assessment of mechanical strength of the treated textiles
The tensile strength of the control and treated fabrics was measured and is shown in Fig. 16. Control cotton fabric shows tensile strength of around 600 N, with a standard deviation of 26 whereas RSB treated cotton fabric depicts the tensile strength of around 695 N (standard deviation of 33). Untreated PET fabric shows a tensile strength of 904 N with a standard deviation of 16, whereas RSB treated PET displays a tensile strength of 830 N with a standard deviation of 20. Tensile testing was performed three times on each sample. Such findings are due to the basicity of RSB, which strengthens the cotton structure to some extent, whereas minor deterioration in tensile strength of treated PET fabric is due to hydrolysis of the PET structure in basic condition.
Table 3 represents the comparative summary of functional properties of the treated textiles using different functional agents. According to the table, reduced Schiff base demonstrates excellent multifunctional efficacy such as UPF, mild fragrance, antimicrobial and antioxidant activities on both the cotton and PET fabrics.
Conclusions
Present research article reveals that RSB, a vanillin derivative (i.e. reduced Schiff base of vanillin), has enormous potential to be used as a biocompatible and safe finishing agent for cotton and PET textiles. Vanillin, on its own, is ineffective due to its small chemical structure and lacks functional activity in textile substrates because of its low retention capability inside the structure. However, by converting vanillin into RSB, it demonstrates remarkable overall properties such as nearly 90% antimicrobial activity, almost 90% antioxidant activity, and good ultraviolet ray protection, all of which are durable against repeated washing. Presently available many durable finishing agents have limited functional properties on textile substrates and result in loss of basic physical properties of textiles such as decreased tensile strength, tearing strength, etc. due to use of stringent conditions during the treatment process which degrade the polymeric backbone in many cases. But RSB provides multiple functional properties without interfering the physical properties of the treated textiles. The present study demonstrates a new route to imbue molecules with both hydrophobic and hydrophilic characteristics on both types of textiles, such as hydrophilic cotton and hydrophobic PET to impart multifunctional effects. However, because of enhanced hydrophobic character of RSB, it can more effectively be integrated with PET fabric. RSB treated textiles have an appealing coloration as well as a pleasant fragrance, which further add values to the treated textiles. Many other derivatives can be prepared in the future for potential replacement of traditional, non-ecofriendly finishing to resolve the issues associated with effluent treatment and environmental load. Not only cotton and PET fabrics, but these green and sustainable finishing chemicals have enormous potential to be explored for other types of textile materials to ascertain multifunctional effects for a wide range of applications.
References
Abraham DJ, Mehanna AS, Wireko FC (1991) Vanillin, a potential agent for the treatment of sickle cell anemia. Blood 77(6):1334–1341
Ahmed HB, Abualnaja KM, Ghareeb RY (2021) Technical textiles modified with immobilized carbon dots synthesized with infrared assistance. J Colloid Interface Sci 604:15–29
Ali SW, Sharma V (2022) Drug nanocrystals: emerging trends in pharmaceutical industries. Ind Appl Nanocrystals. https://doi.org/10.1016/B978-0-12-824024-3.00005-1
Ali SW, Purwar R, Joshi M, Rajendran S (2014) Antibacterial properties of Aloe vera gel-finished cotton fabric. Cellulose 21:2063–2072
Arya SS, Rookes JE, Cahill DM, Lenka SK (2021) Vanillin: a review on the therapeutic prospects of a popular flavouring molecule. Adv Trad Med 21:415–431
Basak S, Wazed Ali S (2018) Fire resistant behaviour of cellulosic textile functionalized with wastage plant bio-molecules: a comparative scientific report. Int J Biol Macromol 114:169–180
Bezerra DP, Soares AKN, De Sousa DP (2016) Overview of the role of vanillin on redox status and cancer development. Oxid Med Cell Longev 2016:1–9
Bezerra FM, Lis MJ, Firmino HB et al (2020) The role of β-cyclodextrin in the textile industry: review. Molecules 25:3624
Chawla M, Sharma V, Randhawa JK (2017) Facile one pot synthesis of CuO nanostructures and their effect on nonenzymatic glucose biosensing. Electrocatalysis 8:27–35
Dhiman G, Chakraborty JN (2015) Antimicrobial performance of cotton finished with triclosan, silver and chitosan. Fash Text 2:1–14
Durrani H, Sharma V, Bamboria D et al (2020) Exploration of flame retardant efficacy of cellulosic fabric using in-situ synthesized zinc borate particles. Cellulose 27:9061–9073
Emam HE, El-Rafie MH, Rehan M (2021a) Functionalization of unbleached flax fibers by direct integration of nano-silver through internal and external reduction. Fibers Polym 22:3014–3024
Emam HE, El-Shahat M, Hasanin MS, Ahmed HB (2021b) Potential military cotton textiles composed of carbon quantum dots clustered from 4–(2, 4–dichlorophenyl)–6–oxo–2–thioxohexahydropyrimidine–5–carbonitrile. Cellulose 28:9991–10011
Emam HE, El-Shahat M, Taha M, Abdelhameed RM (2022a) Microwave assisted post-synthetic modification of IRMOF-3 and MIL-68-NH2 onto cotton for fuel purification with computational explanation. J Surf Interfac 30:101940
Emam HE, Zaghloul S, Ahmed HB (2022b) Full ultraviolet shielding potency of highly durable cotton via self-implantation of palladium nanoclusters. Cellulose 29:4787–4804
Gallage NJ, Møller BL (2017) Vanilla: The most popular flavour. Biotechnol Nat Prod. https://doi.org/10.1007/978-3-319-67903-7_1
Gressier P, De Smet D, Behary N et al (2019) Antibacterial polyester fabrics via diffusion process using active bio-based agents from essential oils. Ind Crops Prod 136:11–20
Hannemann A, Cytlak UMC, Gbotosho OT (2014) Effects of o-vanillin on K+ transport of red blood cells from patients with sickle cell disease. Blood Cells Mol Dis 53(1–2):21–26
Hasanvand E, Rafe A (2019) Development of vanillin/β-cyclodexterin inclusion microcapsules using flax seed gum-rice bran protein complex coacervates. Int J Biol Macromol 131:60–66
Joshi M, Ali SW, Rajendran S (2007) Antibacterial finishing of polyester/cotton blend fabrics using neem (Azadirachta indica): a natural bioactive agent. J Appl Polym Sci 106:793–800
Ketema A, Worku A (2020) Antibacterial finishing of cotton fabric using stinging nettle (Urtica dioica L.) plant leaf extract. J Chem 2020:1–10
Ma W, Zhang Q, Li X (2020) IPM712, a vanillin derivative as potential antitumor agents, displays better antitumor activity in colorectal cancers cell lines. Eur J Pharm Sci 152:105464
Marae IS, Sharmoukh W, Bakhite EA (2021) Durable fluorescent cotton textile by immobilization of unique tetrahydrothienoisoquinoline derivatives. Cellulose 28:5937–5956
Mohajerani A, Ukwatta A, Jeffrey-Bailey T (2019) A proposal for recycling the world’s unused stockpiles of treated wastewater sludge (biosolids) in fired-clay bricks. Buildings 9:14
Pasquet V, Perwuelz A, Behary N, Isaad J (2013) Vanillin, a potential carrier for low temperature dyeing of polyester fabrics. J Clean Prod 43:20–26
Rajpoot Y, Sharma V, Basak S, Ali W (2021) Calcium borate particles: synthesis and application on the cotton fabric as an emerging fire retardant. J Nat Fibers 19(13):5663–5675
Said MM, Rehan M, El-Sheikh SM (2021) Multifunctional hydroxyapatite/silver nanoparticles/cotton gauze for antimicrobial and biomedical applications. Nanomaterials 11:429
Scipioni M, Kay G, Megson I (2018) Novel vanillin derivatives: synthesis, anti-oxidant, DNA and cellular protection properties. Eur J Med Chem 143:745–754
Sharma V, Ali SW (2022a) A unique approach for functionalization of cotton substrate with sustainable bio-based vanillin for multifunctional effects. J Nat Fibers 19(16):14158–14170
Sharma V, Ali SW (2022b) A greener approach to impart multiple functionalities on polyester fabric using Schiff base of vanillin and benzyl amine. Sustain Chem Pharm 27:100645
Sharma V, Chawla M, Randhawa JK (2016) Enhanced sensitivity of nanostructured copper oxide for non-enzymatic glucose biosensing. J Electrochem Soc 163(13):594–600
Sharma V, Basak S, Rishabh K et al (2018) Synthesis of zinc carbonate nanoneedles, a potential flame retardant for cotton textiles. Cellulose 25:6191–6205
Shukla A, Sharma V, Basak S, Ali SW (2019) Sodium lignin sulfonate: a bio-macromolecule for making fire retardant cotton fabric. Cellulose 26(13):8191–8208
Sinha AK, Sharma UK, Sharma N (2009) A comprehensive review on vanilla flavor: extraction, isolation and quantification of vanillin and others constituents. Int J Food Sci Nutr 59:299–326
Štular D, Savio E, Simončič B (2021) Multifunctional antibacterial and ultraviolet protective cotton cellulose developed by in situ biosynthesis of silver nanoparticles into a polysiloxane matrix mediated by sumac leaf extract. Appl Surf Sci 563:150361
Tai A, Sawano T, Yazama F (2011) Antioxidant properties of ethyl vanillin in vitro and in vivo. Biosci Biotechnol Biochem 75:2346–2350
Textile O (2010) The fibre year 2009/10: A world survey on textile and non-woven industry
Thirunavukkarasu K, Rajkumar P, Selvaraj S (2018) Vibrational (FT-IR and FT-Raman), electronic (UV–Vis), NMR (1H and 13C) spectra and molecular docking analyses of anticancer molecule 4-hydroxy-3-methoxycinnamaldehyde. J Mol Struct 1173:307–320
Venkata S, Zeeshan F, Kamal A, Saif F (2020) Efficiency of vanillin in impeding metabolic adaptability and virulence of Candida albicans by inhibiting glyoxylate cycle, morphogenesis, and biofilm formation. Curr Med Mycol 6(1):1–8
Venkata S, Zeeshan F, Saif H (2021) Mechanistic insights into the anticandidal action of Vanillin reveal disruption of cell surface integrity and mitochondrial functioning. Infect Disord Drug Targets 21(3):405–415
Wu Q, Cai H, Yuan T (2020) Novel vanillin derivatives containing a 1,3,4-thiadiazole moiety as potential antibacterial agents. Bioorg Med Chem Lett 30:127113
Funding
The authors would like to express their gratitude to ‘Department of Science and Technology, Government of India’ for the funding support of the research project (DST/TDT/SHRI-08/2018) under the scheme "Science and Heritage Research Initiative" (SHRI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest for publication of this article in this journal.
Human and animal rights
This research article does not involve any human participants and/or animals for studies by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, V., Ali, S.W. Functionalization of cellulosic and polyester textiles using reduced Schiff base (RSB) of eco-friendly vanillin. Cellulose 30, 3317–3338 (2023). https://doi.org/10.1007/s10570-023-05085-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05085-z