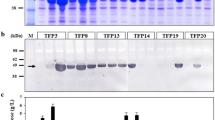
Abstract
The depolymerization of xylo-oligosaccharides to xylose by β-xylosidase and the subsequent ethanol production from xylose are important for reducing inhibition towards cellulase and hemicellulase and for taking full advantage of all sugars in lignocellulose hydrolysate. The activity of β-xylosidase in most cellulases produced by filamentous fungi is usually deficient, and thus recombinant xylose-fermenting Saccharomyces cerevisiae capable of secreting highly active β-xylosidase is increasingly recognized as an excellent candidate for addressing this issue. To this end, a prominent chassis cell, BSPX042, constructed in our previous work with a well-modified downstream metabolic pathway of xylose through multiple chromosome manipulations and beneficial mutations after evolution engineering, was selected as the host. The xylose isomerase coding gene Ru-xylA (where Ru represents the rumen), which was screened from the metagenomics library of bovine rumen contents, was shown to exhibit a high activity level in S. cerevisiae, and the glycoside hydrolase family 3 β-xylosidase gene xyl3A from Penicillium oxalicum was coexpressed in BSPX042. The recombinant strain BSGIBX with the highest extracellular activity of β-xylosidase was determined by comparing different signal peptides. The hydrolysis of xylobiose and xylotriose by BSGIBX culture further confirmed its β-xylosidase activity. BSGIBX can grow and produce ethanol with xylo-oligosaccharides as the sole carbon source. When xylo-oligosaccharides were pretreated by effective xylanase, the majority of the xylo-oligosaccharides were consumed rapidly and produced ~ 19.4 g L−1 ethanol at 48 h with a yield of 0.473 g g−1 consumed sugars in mixture with glucose. These results indicate the successful coexpression of β-xylosidase and xylose isomerase in ethanol-producing S. cerevisiae, and provide theoretical support for our future work in robust industrial yeast strains.





Similar content being viewed by others
References
Banerjee G, Car S, Scott-Craig JS, Borrusch MS, Walton JD (2010) Rapid optimization of enzyme mixtures for deconstruction of diverse pretreatment/biomass feedstock combinations. Biotechnol Biofuels. https://doi.org/10.1186/1754-6834-3-22
Bastawde KBY (1992) Xylan structure, microbial xylanases, and their mode of action. World J Microbiol Biotechnol 8:353–368. https://doi.org/10.1007/BF01198746
Cagnon B, Py X, Guillot A, Stoeckli F, Chambat G (2009) Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour Technol 100:292–298. https://doi.org/10.1016/j.biortech.2008.06.009
Chen Y, Daviet L, Schalk M, Siewers V, Nielsen J (2013) Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metab Eng 15:48–54. https://doi.org/10.1016/j.ymben.2012.11.002
Fujii T, Yu G, Matsushika A, Kurita A, Yano S, Murakami K, Sawayama S (2011) Ethanol production from xylo-oligosaccharides by xylose-fermenting Saccharomyces cerevisiae expressing beta-xylosidase. Biosci Biotechnol Biochem 75:1140–1146. https://doi.org/10.1271/bbb.110043
Fujitomi K, Sanda T, Hasunuma T, Kondo A (2012) Deletion of the PHO13 gene in Saccharomyces cerevisiae improves ethanol production from lignocellulosic hydrolysate in the presence of acetic and formic acids, and furfural. Bioresour Technol 111:161–166. https://doi.org/10.1016/j.biortech.2012.01.161
Gao M, Ploessl D, Shao Z (2018) Enhancing the Co-utilization of biomass-derived mixed sugars by yeasts. Front Microbiol 9:3264. https://doi.org/10.3389/fmicb.2018.03264
Gibson DG (2011) Enzymatic assembly of overlapping DNA fragments. Method Enzymol 498:349–361. https://doi.org/10.1016/B978-0-12-385120-8.00015-2
Gietz RD, Woods RA (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350:87–96. https://doi.org/10.1016/S0076-6879(02)50957-5
Hou J, Jiao C, Peng B, Shen Y, Bao X (2016a) Mutation of a regulator Ask10p improves xylose isomerase activity through up-regulation of molecular chaperones in Saccharomyces cerevisiae. Metab Eng 38:241–250. https://doi.org/10.1016/j.ymben.2016.08.001
Hou J, Shen Y, Jiao C, Ge R, Zhang X, Bao X (2016b) Characterization and evolution of xylose isomerase screened from the bovine rumen metagenome in Saccharomyces cerevisiae. J Biosci Bioeng 121:160–165. https://doi.org/10.1016/j.jbiosc.2015.05.014
Kabel MA, Schols HA, Voragen AGJ (2002) Complex xylo-oligosaccharides identified from hydrothermally treated Eucalyptus wood and brewery’s spent grain. Carbohydr Polym 50(2):191–200. https://doi.org/10.1016/S0144-8617(02)00022-X
Katahira S, Fujita Y, Mizuike A, Fukuda H, Kondo A (2004) Construction of a xylan-fermenting yeast strain through codisplay of xylanolytic enzymes on the surface of xylose-utilizing Saccharomyces cerevisiae cells. Appl Environ Microb 70:5407–5414. https://doi.org/10.1128/AEM.70.9.5407-5414.2004
Kim SR, Skerker JM, Kang W, Lesmana A, Wei N, Arkin AP, Jin YS (2013) Rational and evolutionary engineering approaches uncover a small set of genetic changes efficient for rapid xylose fermentation in Saccharomyces cerevisiae. PLoS ONE. https://doi.org/10.1371/journal.pone.0057048
Kirikyali N, Connerton IF (2014) Heterologous expression and kinetic characterisation of Neurospora crassa beta-xylosidase in Pichia pastoris. Enzyme Microb Technol 57:63–68. https://doi.org/10.1016/j.enzmictec.2014.02.002
Kumar R, Wyman CE (2009) Effect of xylanase supplementation of cellulase on digestion of corn stover solids prepared by leading pretreatment technologies. Bioresour Technol 100:4203–4213. https://doi.org/10.1016/j.biortech.2008.11.057
Kwak S, Jin YS (2017) Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: a review and perspective. Microb Cell Fact 16:82. https://doi.org/10.1186/s12934-017-0694-9
La Grange DC, Pretorius IS, Claeyssens M, van Zyl WH (2001) Degradation of xylan to d-xylose by recombinant Saccharomyces cerevisiae coexpressing the Aspergillus niger beta-xylosidase (xlnD) and the Trichoderma reesei xylanase II (xyn2) genes. Appl Environ Microbiol 67:5512–5519. https://doi.org/10.1128/AEM.67.12.5512-5519.2001
Lee S-M, Jellison T, Alper HS (2014) Systematic and evolutionary engineering of a xylose isomerase-based pathway in Saccharomyces cerevisiae for efficient conversion yields. Biotechnol Biofuels 7:122. https://doi.org/10.1186/s13068-014-0122-x
Li X, Yu VY, Lin Y, Chomvong K, Estrela R, Park A, Liang JM, Znameroski EA, Feehan J, Kim SR (2015) Expanding xylose metabolism in yeast for plant cell wall conversion to biofuels. Elife 4:e05896. https://doi.org/10.7554/eLife.05896.001
Li H, Shen Y, Wu M, Hou J, Jiao C, Li Z, Liu X, Bao X (2016a) Engineering a wild-type diploid Saccharomyces cerevisiae strain for second-generation bioethanol production. Bioresour Bioprocess 3:51. https://doi.org/10.1186/s40643-016-0126-4
Li HB, Schmitz O, Alper HS (2016b) Enabling glucose/xylose co-transport in yeast through the directed evolution of a sugar transporter. Appl Microbiol Biot 100:10215–10223. https://doi.org/10.1007/s00253-016-7879-8
Li X, Chen Y, Nielsen J (2019) Harnessing xylose pathways for biofuels production. Curr Opin Biotechnol 57:56–65. https://doi.org/10.1016/j.copbio.2019.01.006
Liu G, Zhang Q, Li H, Qureshi AS, Zhang J, Bao X, Bao J (2018a) Dry biorefining maximizes the potentials of simultaneous saccharification and co-fermentation for cellulosic ethanol production. Biotechnol Bioeng 115:60–69. https://doi.org/10.1002/bit.26444
Liu T, Huang S, Geng A (2018b) Recombinant diploid Saccharomyces cerevisiae strain development for rapid glucose and xylose co-fermentation. Fermentation 4:59
Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R, Himmel M, Keller M, McMillan JD, Sheehan JJNB (2008) How biotech can transform biofuels. Nat Biotechnol 26:169. https://doi.org/10.1038/nbt0208-169
Margolles-Clark E, Tenkanen M, Nakari-Setala T, Penttila M (1996) Cloning of genes encoding alpha-l-arabinofuranosidase and beta-xylosidase from Trichoderma reesei by expression in Saccharomyces cerevisiae. Appl Environ Microbiol 62:3840–3846
Mert MJ, la Grange DC, Rose SH, van Zyl WH (2016) Engineering of Saccharomyces cerevisiae to utilize xylan as a sole carbohydrate source by co-expression of an endoxylanase, xylosidase and a bacterial xylose isomerase. J Ind Microbiol Biot 43:431–440. https://doi.org/10.1007/s10295-015-1727-1
Peng B, Shen Y, Li X, Chen X, Hou J, Bao X (2012) Improvement of xylose fermentation in respiratory-deficient xylose-fermenting Saccharomyces cerevisiae. Metab Eng 14:9–18. https://doi.org/10.1016/j.ymben.2011.12.001
Peng S, Cao Q, Qin Y, Li X, Liu G, Qu Y (2017) An aldonolactonase AltA from Penicillium oxalicum mitigates the inhibition of beta-glucosidase during lignocellulose biodegradation. Appl Microbiol Biotechnol 101:3627–3636. https://doi.org/10.1007/s00253-017-8134-7
Qing Q, Wyman CE (2011a) Hydrolysis of different chain length xylooliogmers by cellulase and hemicellulase. Bioresour Technol 102:1359–1366. https://doi.org/10.1016/j.biortech.2010.09.001
Qing Q, Wyman CE (2011b) Supplementation with xylanase and beta-xylosidase to reduce xylo-oligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover. Biotechnol Biofuels 4:18. https://doi.org/10.1186/1754-6834-4-18
Runquist D, Hahn-Hägerdal B, Bettiga M (2010) Increased ethanol productivity in xylose-utilizing Saccharomyces cerevisiae via a randomly mutagenized xylose reductase. Appl Environ Microbiol 76:7796–7802. https://doi.org/10.1128/AEM.01505-10
Saitoh S, Tanaka T, Kondo A (2011) Co-fermentation of cellulose/xylan using engineered industrial yeast strain OC-2 displaying both beta-glucosidase and beta-xylosidase. Appl Microbiol Biot 91:1553–1559. https://doi.org/10.1007/s00253-011-3357-5
Sakamoto T, Hasunuma T, Hori Y, Yamada R, Kondo A (2012) Direct ethanol production from hemicellulosic materials of rice straw by use of an engineered yeast strain codisplaying three types of hemicellulolytic enzymes on the surface of xylose-utilizing Saccharomyces cerevisiae cells. J Biotechnol 158:203–210. https://doi.org/10.1016/j.jbiotec.2011.06.025
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27. https://doi.org/10.1016/j.renene.2011.06.045
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289. https://doi.org/10.1146/annurev-arplant-042809-112315
Shen Y, Chen X, Peng B, Chen L, Hou J, Bao X (2012) An efficient xylose-fermenting recombinant Saccharomyces cerevisiae strain obtained through adaptive evolution and its global transcription profile. Appl Microbiol Biotechnol 96:1079–1091. https://doi.org/10.1007/s00253-012-4418-0
Tang H, Hou J, Shen Y, Xu L, Yang H, Fang X, Bao X (2013) High β-glucosidase secretion in Saccharomyces cerevisiae improves the efficiency of cellulase hydrolysis and ethanol production in simultaneous saccharification and fermentation. J Microbiol Biotechnol 23:1577–1585. https://doi.org/10.4014/jmb.1305.05011
van Rensburg E, den Haan R, Smith J, van Zyl WH, Gorgens JF (2012) The metabolic burden of cellulase expression by recombinant Saccharomyces cerevisiae Y294 in aerobic batch culture. Appl Microbiol Biotechnol 96:197–209. https://doi.org/10.1007/s00253-012-4037-9
Van Vleet JH, Jeffries TW, Olsson L (2008) Deleting the para-nitrophenyl phosphatase (pNPPase), PHO13, in recombinant Saccharomyces cerevisiae improves growth and ethanol production on d-xylose. Metab Eng 10:360–369. https://doi.org/10.1016/j.ymben.2007.12.002
Wang C, Bao X, Li Y, Jiao C, Hou J, Zhang Q, Zhang W, Liu W, Shen Y (2015) Cloning and characterization of heterologous transporters in Saccharomyces cerevisiae and identification of important amino acids for xylose utilization. Metab Eng 30:79–88. https://doi.org/10.1016/j.ymben.2015.04.007
Wang M, Yu CZ, Zhao HM (2016) Directed evolution of xylose specific transporters to facilitate glucose–xylose co-utilization. Biotechnol Bioeng 113:484–491. https://doi.org/10.1002/bit.25724
Wang C, Lu X, Gao J, Li X, Zhao JJE (2018) Xylo-oligosaccharides inhibit enzymatic hydrolysis by influencing enzymatic activity of cellulase from Penicillium oxalicum. Energy Fuels 32:9427–9437. https://doi.org/10.1021/acs.energyfuels.8b01424
Wei N, Quarterman J, Kim SR, Cate JHD, Jin Y-S (2013) Enhanced biofuel production through coupled acetic acid and xylose consumption by engineered yeast. Nat Commun 4:2580. https://doi.org/10.1038/ncomms3580
Wei S, Liu Y, Wu M, Ma T, Bai X, Hou J, Shen Y, Bao X (2018) Disruption of the transcription factors Thi2p and Nrm1p alleviates the post-glucose effect on xylose utilization in Saccharomyces cerevisiae. Biotechnol Biofuels 11:112. https://doi.org/10.1186/s13068-018-1112-1
Xin D, Sun Z, Viikari L, Zhang J (2015) Role of hemicellulases in production of fermentable sugars from corn stover. Ind Crop Prod 74:209–217. https://doi.org/10.1016/j.indcrop.2015.05.017
Ye Y, Li X, Cao Y, Du J, Chen S, Zhao J (2017a) A beta-xylosidase hyper-production Penicillium oxalicum mutant enhanced ethanol production from alkali-pretreated corn stover. Bioresour Technol 245:734–742. https://doi.org/10.1016/j.biortech.2017.08.155
Ye Y, Li X, Zhao J (2017b) Production and characteristics of a novel Xylose-and Alkali-tolerant GH 43 β-xylosidase from Penicillium oxalicum for promoting hemicellulose degradation. Sci Rep 7:11600. https://doi.org/10.1038/s41598-017-11573-7
Young EM, Tong A, Bui H, Spofford C, Alper HS (2014) Rewiring yeast sugar transporter preference through modifying a conserved protein motif. Proc Nat Acad Sci 111:131–136. https://doi.org/10.1073/pnas.1311970111
Zhang J, Tang M, Viikari L (2012) Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases. Bioresour Technol 121:8–12. https://doi.org/10.1016/j.biortech.2012.07.010
Zhou H, Cheng J-S, Wang BL, Fink GR, Stephanopoulos G (2012) Xylose isomerase overexpression along with engineering of the pentose phosphate pathway and evolutionary engineering enable rapid xylose utilization and ethanol production by Saccharomyces cerevisiae. Metab Eng 14:611–622. https://doi.org/10.1016/j.ymben.2012.07.011
Acknowledgments
This work was supported by the National Key R&D Program of China (2018YFB1501401, 2018YFB1501702), the National Natural Science Foundation of China (31870063), the Key R&D Project of Shandong Province (2017CXGC1105), the Major Program of Shandong Province Natural Science Foundation (ZR2018ZB0209) and the China Postdoctoral Science Foundation (2019M652374).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaping Niu and Longhao Wu have contributed equally to this work and should be considered as co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
The fermentation chromatograms of strain BSGIBX in SC-X. (PPTX 56 kb)
Rights and permissions
About this article
Cite this article
Niu, Y., Wu, L., Shen, Y. et al. Coexpression of β-xylosidase and xylose isomerase in Saccharomyces cerevisiae improves the efficiency of saccharification and fermentation from xylo-oligosaccharides. Cellulose 26, 7923–7937 (2019). https://doi.org/10.1007/s10570-019-02650-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02650-3