Abstract
Background
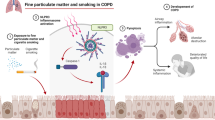
Long-term exposure to PM2.5 (particulate matter with an aerodynamic diameter of ≤ 2.5 μm) is associated with pulmonary injury and emphysema in patients with chronic obstructive pulmonary disease (COPD). We investigated mechanisms through which the long noncoding RNA lnc-IL7R contributes to cellular damage by inducing oxidative stress in COPD patients exposed to PM2.5.
Methods
Associations of serum lnc-IL7R levels with lung function, emphysema, and previous PM2.5 exposure in COPD patients were analyzed. Reactive oxygen species and lnc-IL7R levels were measured in PM2.5-treated cells. The levels of lnc-IL7R and cellular senescence–associated genes, namely p16INK4a and p21CIP1/WAF1, were determined through lung tissue section staining. The effects of p16INK4a or p21CIP1/WAF1 regulation were examined by performing lnc-IL7R overexpression and knockdown assays. The functions of lnc-IL7R-mediated cell proliferation, cell cycle, senescence, colony formation, and apoptosis were examined in cells treated with PM2.5. Chromatin immunoprecipitation assays were conducted to investigate the epigenetic regulation of p21CIP1/WAF1.
Results
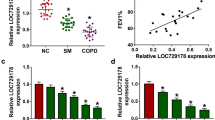
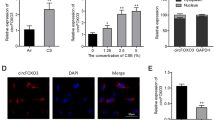
Lnc-IL7R levels decreased in COPD patients and were negatively correlated with emphysema or PM2.5 exposure. Lnc-IL7R levels were upregulated in normal lung epithelial cells but not in COPD cells exposed to PM2.5. Lower lnc-IL7R expression in PM2.5-treated cells induced p16INK4a and p21CIP1/WAF1 expression by increasing oxidative stress. Higher lnc-IL7R expression protected against cellular senescence and apoptosis, whereas lower lnc-IL7R expression augmented injury in PM2.5-treated cells. Lnc-IL7R and the enhancer of zeste homolog 2 (EZH2) synergistically suppressed p21CIP1/WAF1 expression through epigenetic modulation.
Conclusion
Lnc-IL7R attenuates PM2.5-mediated p21CIP1/WAF1 expression through EZH2 recruitment, and its dysfunction may augment cellular injury in COPD.
Graphical abstract









Similar content being viewed by others
Availability of data and material
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Differential genes were analyzed in published microarray data sets.
References
Barnes PJ. Senescence in COPD and its comorbidities. Annu Rev Physiol. 2017;79:517–39. https://doi.org/10.1146/annurev-physiol-022516-034314.
Barnes PJ, Baker J, Donnelly LE. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med. 2019;200(5):556–64. https://doi.org/10.1164/rccm.201810-1975TR.
Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1:15076. https://doi.org/10.1038/nrdp.2015.76.
Bo Y, Chang LY, Guo C, Lin C, Lau AKH, Tam T, et al. Reduced ambient PM2.5, better lung function, and decreased risk of chronic obstructive pulmonary disease. Environ Int. 2021;156:106706. https://doi.org/10.1016/j.envint.2021.106706.
Brandsma CA, de Vries M, Costa R, Woldhuis RR, Konigshoff M, Timens W. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair? Eur Respir Rev. 2017;26(146). https://doi.org/10.1183/16000617.0073-2017.
Chen TT, Wu SM, Ho SC, Chuang HC, Liu CY, Chan YF, et al. SUV39H1 reduction is implicated in abnormal inflammation in COPD. Sci Rep. 2017;7:46667. https://doi.org/10.1038/srep46667.
Chen XY, Feng PH, Han CL, Jheng YT, Wu CD, Chou HC, et al. Alveolar epithelial inter-alpha-trypsin inhibitor heavy chain 4 deficiency associated with senescence-regulated apoptosis by air pollution. Environ Pollut. 2021;278:116863. https://doi.org/10.1016/j.envpol.2021.116863.
Cui H, Xie N, Tan Z, Banerjee S, Thannickal VJ, Abraham E, et al. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. Eur J Immunol. 2014;44(7):2085–95. https://doi.org/10.1002/eji.201344126.
Devadoss D, Long C, Langley RJ, Manevski M, Nair M, Campos MA, et al. Long noncoding transcriptome in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2019;61(6):678–88. https://doi.org/10.1165/rcmb.2019-0184TR.
Doiron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J. 2019;54(1):1802140. https://doi.org/10.1183/13993003.02140-2018.
Ferrari L, Carugno M, Bollati V. Particulate matter exposure shapes DNA methylation through the lifespan. Clin Epigenetics. 2019;11(1):129. https://doi.org/10.1186/s13148-019-0726-x.
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD (2020 report). 2020.
Guo C, Zhang Z, Lau AKH, Lin CQ, Chuang YC, Chan J, et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health. 2018;2(3):e114–25. https://doi.org/10.1016/S2542-5196(18)30028-7.
Hezroni H, Koppstein D, Schwartz MG, Avrutin A, Bartel DP, Ulitsky I. Principles of long noncoding RNA evolution derived from direct comparison of transcriptomes in 17 species. Cell Rep. 2015;11(7):1110–22. https://doi.org/10.1016/j.celrep.2015.04.023.
Hoffman EA, Ahmed FS, Baumhauer H, Budoff M, Carr JJ, Kronmal R, et al. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc. 2014;11(6):898–907. https://doi.org/10.1513/AnnalsATS.201310-364OC.
Huang HC, Lin FC, Wu MF, Nfor ON, Hsu SY, Lung CC, et al. Association between chronic obstructive pulmonary disease and PM2.5 in Taiwanese nonsmokers. Int J Hyg Environ Health. 2019;222(5):884–8. https://doi.org/10.1016/j.ijheh.2019.03.009.
Huang Q, Chi Y, Deng J, Liu Y, Lu Y, Chen J, et al. Fine particulate matter 2.5 exerted its toxicological effect by regulating a new layer, long non-coding RNA. Sci Rep. 2017;7(1):9392. https://doi.org/10.1038/s41598-017-09818-6.
Huggins FE, Huffman GP, Robertson JD. Speciation of elements in NIST particulate matter SRMs 1648 and 1650. J Hazard Mater. 2000;74(1–2):1–23. https://doi.org/10.1016/s0304-3894(99)00195-8.
Jin X, Xue B, Zhou Q, Su R, Li Z. Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM2.5 exposure. J Toxicol Sci. 2018;43(2):101–11. https://doi.org/10.2131/jts.43.101.
Kuznar-Kaminska B, Mikula-Pietrasik J, Witucka A, Romaniuk A, Konieczna N, Rubis B, et al. Serum from patients with chronic obstructive pulmonary disease induces senescence-related phenotype in bronchial epithelial cells. Sci Rep. 2018;8(1):12940. https://doi.org/10.1038/s41598-018-31037-w.
Lee H, Hwang-Bo H, Ji SY, Kim MY, Kim SY, Park C, et al. Diesel particulate matter 2.5 promotes epithelial-mesenchymal transition of human retinal pigment epithelial cells via generation of reactive oxygen species. Environ Pollut. 2020;262:114301. https://doi.org/10.1016/j.envpol.2020.114301.
Lee JW, Lee HJ, Lee YJ, Lim YB, Sim WJ, Jang JH, et al. Determination of genotoxicity attributed to diesel exhaust particles in normal human embryonic lung cell (WI-38) line. Biomolecules. 2021;11(2). https://doi.org/10.3390/biom11020291.
Lee YL, Chen JH, Wang CM, Chen ML, Hwang BF. Association of air pollution exposure and interleukin-13 haplotype with the risk of aggregate bronchitic symptoms in children. EBioMedicine. 2018;29:70–7. https://doi.org/10.1016/j.ebiom.2018.02.008.
Li R, Zhou R, Zhang J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol Lett. 2018a;15(5):7506–14. https://doi.org/10.3892/ol.2018.8355.
Li X, Zheng M, Pu J, Zhou Y, Hong W, Fu X, et al. Identification of abnormally expressed lncRNAs induced by PM2.5 in human bronchial epithelial cells. Biosci Rep. 2018b;38(5). https://doi.org/10.1042/BSR20171577.
Longhin E, Holme JA, Gutzkow KB, Arlt VM, Kucab JE, Camatini M, et al. Cell cycle alterations induced by urban PM2.5 in bronchial epithelial cells: characterization of the process and possible mechanisms involved. Part Fibre Toxicol. 2013;10:63. https://doi.org/10.1186/1743-8977-10-63.
Lutchmedial SM, Creed WG, Moore AJ, Walsh RR, Gentchos GE, Kaminsky DA. How common is airflow limitation in patients with emphysema on CT scan of the chest? Chest. 2015;148(1):176–84. https://doi.org/10.1378/chest.14-1556.
MacNee W. Is chronic obstructive pulmonary disease an accelerated aging disease? Ann Am Thorac Soc. 2016;13 Suppl 5:S429–37. https://doi.org/10.1513/AnnalsATS.201602-124AW.
Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176(3):243–52. https://doi.org/10.1164/rccm.200606-828OC.
McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–75. https://doi.org/10.1056/NEJMoa1106955.
McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369(10):910–9. https://doi.org/10.1056/NEJMoa1214726.
Mijit M, Caracciolo V, Melillo A, Amicarelli F, Giordano A. Role of p53 in the regulation of cellular senescence. Biomolecules. 2020;10(3). https://doi.org/10.3390/biom10030420.
Nemec SF, Bankier AA, Eisenberg RL. Upper lobe–predominant diseases of the lung. Am J Roentgenol. 2013;200(3):W222–37. https://doi.org/10.2214/AJR.12.8961.
Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–41. https://doi.org/10.1016/j.cell.2009.02.006.
Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015;191(6):656–64. https://doi.org/10.1164/rccm.201410-1875OC.
Rodriguez-Roisin R, Rabe KF, Vestbo J, Vogelmeier C, Agusti A, all p, et al. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 20th anniversary: a brief history of time. Eur Respir J. 2017;50(1). https://doi.org/10.1183/13993003.00671-2017.
Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM, Bossis G, et al. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp Mol Med. 2019a;51(9):1–14. https://doi.org/10.1038/s12276-019-0305-4.
Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM, Hyun YM, et al. Particulate matter induces inflammatory cytokine production via activation of NFkappaB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019b;21:101080. https://doi.org/10.1016/j.redox.2018.101080.
Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138(1):3–6. https://doi.org/10.1378/chest.10-0645.
Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–43. https://doi.org/10.1016/S0140-6736(09)61303-9.
Shaikh M, Sood RG, Sarkar M, Thakur V. Quantitative computed tomography (CT) assessment of emphysema in patients with severe chronic obstructive pulmonary disease (COPD) and its correlation with age, sex, pulmonary function tests, BMI, smoking, and biomass exposure. Pol J Radiol. 2017;82:760–6. https://doi.org/10.12659/PJR.903278.
Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of “overspill” of inflammatory mediators from the lungs? Review of the evidence. Thorax. 2010;65(10):930–6. https://doi.org/10.1136/thx.2009.130260.
Smith BM, Austin JHM, Newell JD Jr, D’Souza BM, Rozenshtein A, Hoffman EA, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014;127(1):94.e7. https://doi.org/10.1016/j.amjmed.2013.09.020.
Sorheim IC, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–5. https://doi.org/10.1136/thx.2009.122002.
Sousa C, Rodrigues M, Carvalho A, Viamonte B, Cunha R, Guimarães S, et al. Diffuse smoking-related lung diseases: insights from a radiologic-pathologic correlation. Insights Imaging. 2019;10(1):73. https://doi.org/10.1186/s13244-019-0765-z.
Tsai IC, Lee CY, Lung SC, Su CW. Characterization of the vehicle emissions in the Greater Taipei Area through vision-based traffic analysis system and its impacts on urban air quality. Sci Total Environ. 2021;782:146571. https://doi.org/10.1016/j.scitotenv.2021.146571.
Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–33. https://doi.org/10.1093/nar/gkr254.
Vlahovic G, Russell ML, Mercer RR, Crapo JD. Cellular and connective tissue changes in alveolar septal walls in emphysema. Am J Respir Crit Care Med. 1999;160(6):2086–92. https://doi.org/10.1164/ajrccm.160.6.9706031.
Wan B, Xu WJ, Xu WN, Zhan P, Wu GN, Jin JJ, et al. Plasma long noncoding RNA IL-7R as a prognostic biomarker for clinical outcomes in patients with acute respiratory distress syndrome. Clin Respir J. 2018;12(4):1607–14. https://doi.org/10.1111/crj.12717.
Wang M, Aaron CP, Madrigano J, Hoffman EA, Angelini E, Yang J, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA. 2019;322(6):546–56. https://doi.org/10.1001/jama.2019.10255.
Wang N, Wang Q, Du T, Gabriel ANA, Wang X, Sun L, et al. The potential roles of exosomes in chronic obstructive pulmonary disease. Front Med (Lausanne). 2020;7:618506. https://doi.org/10.3389/fmed.2020.618506.
Wu SM, Feng PH, Chuang HC, Ho SC, Fan Chung K, Chen KY, et al. Impaired lnc-IL7R modulatory mechanism of Toll-like receptors is associated with an exacerbator phenotype of chronic obstructive pulmonary disease. FASEB J. 2020;34(10):13317–32. https://doi.org/10.1096/fj.202000632R.
Wu SM, Sun WL, Lee KY, Lin CW, Feng PH, Chuang HC, et al. Determinants of pulmonary emphysema severity in taiwanese patients with chronic obstructive pulmonary disease: an integrated epigenomic and air pollutant analysis. Biomedicines. 2021;9(12). https://doi.org/10.3390/biomedicines9121833.
Xu Y, Wu J, Peng X, Yang T, Liu M, Chen L, et al. LncRNA LINC00341 mediates PM2.5-induced cell cycle arrest in human bronchial epithelial cells. Toxicol Lett. 2017;276:1–10. https://doi.org/10.1016/j.toxlet.2017.03.026.
Yang X, Zhang T, Zhang Y, Chen H, Sang S. Global burden of COPD attributable to ambient PM2.5 in 204 countries and territories, 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Sci Total Environ. 2021;796:148819. https://doi.org/10.1016/j.scitotenv.2021.148819.
Ye Z, Xu J, Li S, Cai C, Li T, Sun L. LncIL7R promotes the growth of fibroblastlike synoviocytes through interaction with enhancer of zeste homolog 2 in rheumatoid arthritis. Mol Med Rep. 2017;15(3):1412–8. https://doi.org/10.3892/mmr.2017.6150.
Yoon YS, Jin M, Sin DD. Accelerated lung aging and chronic obstructive pulmonary disease. Expert Rev Respir Med. 2019;13(4):369–80. https://doi.org/10.1080/17476348.2019.1580576.
Yuan D, Liu Y, Li M, Zhou H, Cao L, Zhang X, et al. Senescence associated long non-coding RNA 1 regulates cigarette smoke-induced senescence of type II alveolar epithelial cells through sirtuin-1 signaling. J Int Med Res. 2021;49(2):300060520986049. https://doi.org/10.1177/0300060520986049.
Yuan X, Wang Y, Li L, Zhou W, Tian D, Lu C, et al. PM2.5 induces embryonic growth retardation: potential involvement of ROS-MAPKs-apoptosis and G0/G1 arrest pathways. Environ Toxicol. 2016;31(12):2028–44. https://doi.org/10.1002/tox.22203.
Zheng M, Hong W, Gao M, Yi E, Zhang J, Hao B, et al. Long noncoding RNA COPDA1 promotes airway smooth muscle cell proliferation in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2019;61(5):584–96. https://doi.org/10.1165/rcmb.2018-0269OC.
Zhou B, Liang G, Qin H, Peng X, Huang J, Li Q, et al. p53-Dependent apoptosis induced in human bronchial epithelial (16-HBE) cells by PM(2.5) sampled from air in Guangzhou, China. Toxicol Mech Methods. 2014;24(8):552–9. https://doi.org/10.3109/15376516.2014.951814.
Zhou Z, Liu Y, Duan F, Qin M, Wu F, Sheng W, et al. Transcriptomic analyses of the biological effects of airborne PM2.5 exposure on human bronchial epithelial cells. PLoS One. 2015;10(9):e0138267. https://doi.org/10.1371/journal.pone.0138267.
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
This study was funded by the Ministry of Science and Technology of Taiwan (MOST: 108–2314-B-038–111-MY3 and 108–2314-B-038–063-MY3), Ministry of Education of the Republic of China (DP2-110–21121-01-T-01–01), and Taipei Medical University and Shuang Ho Hospital (110TMU-SHH-19).
Author information
Authors and Affiliations
Contributions
K. Y. L., S. C. H., and S. M. W conceptualized the study and reviewed the entire project and manuscript. W. L. S. and P. H. F. performed most of the experiments and wrote the manuscript. P. H. F. and C. W. L. designed the research and conducted the experiments. K. Y. C. and H. C. C. contributed to the exposure assessment. C. H. T. provided expertise on statistical and figure analyses. P. H. F., T. T. C., and K. Y. C. conducted patient information analyses and reviewed the manuscript. S. M. W. assumes responsibility for the content of the manuscript, including the data and analysis. All authors contributed to the critical revision of the manuscript for important intellectual content. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All experiments were performed in accordance with relevant guidelines and regulations. The study protocol was approved by the Joint Institutional Review Board of Taipei Medical University.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Low lnc-IL7R expression is associated with emphysema or PM2.5 exposure in COPD.
• Lnc-IL7R expression was upregulated in normal but not COPD lung epithelial cells exposed to PM2.5.
• Lnc-IL7R is a negative regulator of PM2.5-mediated p21CIP1/WAF1 and p16INK4a expression.
• Blockade of lnc-IL7R expression and EZH2 recruitment increases p21CIP1/WAF1 gene expression in cells exposed to PM2.5, which may aggravate cellular injury in COPD.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, KY., Ho, SC., Sun, WL. et al. Lnc-IL7R alleviates PM2.5-mediated cellular senescence and apoptosis through EZH2 recruitment in chronic obstructive pulmonary disease. Cell Biol Toxicol 38, 1097–1120 (2022). https://doi.org/10.1007/s10565-022-09709-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-022-09709-1