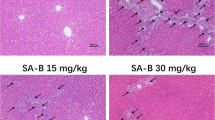
Abstract
Cholestatic liver injury, a group of diseases characterized with dysregulated bile acid (BA) homeostasis, was partly resulted from BA circulation disorders, which is commonly associated with the damage of hepatocyte barrier function. However, the underlying hepatocyte barrier-protective molecular mechanisms of cholestatic liver injury remain poorly understood. Interestingly, recent studies have shown that sphingosine-1-phosphate (S1P) participated in the process of cholestasis by activating its G protein–coupled receptors S1PRs, regaining the integrity of hepatocyte tight junctions (TJs). Here, we showed that SEW2871, a selective agonist of sphingosine-1-phosphate receptor 1(S1PR1), alleviated ANIT–induced TJs damage in 3D-cultured mice primary hepatocytes. Molecular mechanism studies indicated that AMPK signaling pathways was involved in TJs protection of SEW2871 in ANIT-induced hepatobiliary barrier function deficiency. AMPK antagonist compound C (CC) and agonist AICAR were all used to further identify the important role of AMPK signaling pathway in SEW2871’s TJs protection of ANIT-treated mice primary hepatocytes. The in vivo data showed that SEW2871 ameliorated ANIT-induced cholestatic hepatotoxicity. Further protection mechanism research demonstrated that SEW2871 not only regained hepatocyte TJs by the upregulated S1PR1 via AMPK signaling pathway, but also recovered hepatobiliary barrier function deficiency, which was verified by the restored BA homeostasis by using of high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS). These results revealed that the increased expression of S1PR1 induced by SEW2871 could ameliorate ANIT–induced cholestatic liver injury through improving liver barrier function via AMPK signaling and subsequently reversed the disrupted BA homeostasis. Our study provided strong evidence that S1PR1 may be a promising therapeutic approach for treating intrahepatic cholestatic liver injury.

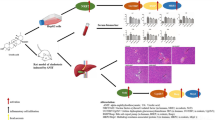
Graphical abstract







Similar content being viewed by others
Abbreviations
- ANIT:
-
α-Naphthylisothiocyanate
- TJs:
-
Tight junctions
- ZO-1:
-
Zonula occludens 1
- S1P:
-
Sphingosine-1-phosphate
- S1PR1:
-
sphingosine-1-phosphate receptor 1
- AMPK:
-
AMP-activated protein kinase
- LKB1:
-
Liver kinase B1
- ERK1/2:
-
Extracellular signal-regulated kinases
- CC:
-
Compound C
- MPO:
-
Myeloperoxidase
- IL-6:
-
Interleukin-6
- IL-1β:
-
Interleukin-1β
- TNF-α:
-
Tumor necrosis factor-α
- SCMHs:
-
Sandwich cultured primary mice hepatocytes
- LC-MS/MS:
-
High-performance liquid chromatography-tandem mass spectrometry
- PCA:
-
Principal component analysis
- G-BAs:
-
Glycine-conjugated bile acids
- T-BAs:
-
Taurine conjugated bile acids
- UDCA:
-
Ursodeoxycholic acid
- HDCA:
-
Hyodeoxycholic acid
- CDCA:
-
Chenodeoxycholic acid
- DCA:
-
Deoxycholic acid
- CA:
-
Cholic acid
- Beta-MCA:
-
Beta-muricholic acid
- LCA:
-
Lithocholic acid
- TUDCA:
-
Tauroursodeoxycholic acid
- THDCA:
-
Taurohyodeoxycholic acid
- TCDCA:
-
Taurochenodeoxycholic acid
- TDCA:
-
Taurodeoxycholic acid
- TCA:
-
Taurocholic acid
- TLCA:
-
Taurolithocholic acid
- GUDCA:
-
Glycoursodeoxycholic acid
- GHDCA:
-
Glycohyodeoxycholic acid
- GCDCA:
-
Glycochenodeoxycholic acid
- GDCA:
-
Glycodeoxycholic acid
- GCA:
-
Glycocholic acid
- GLCA:
-
Glycolithocholic acid
- dhCA:
-
Dehydrocholic acid
- NorUDCA:
-
24-norursodeoxycholic acid
- OCA:
-
Obecholic acid
- EE:
-
17α-Ethinylestradiol
References
Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–77.
Assimakopoulos SF, Tsamandas AC, Louvros E, Vagianos CE, Nikolopoulou VN, Thomopoulos KC, et al. Intestinal epithelial cell proliferation, apoptosis and expression of tight junction proteins in patients with obstructive jaundice. Eur J Clin Investig. 2011;41:117–25.
Aw DK, Sinha RA, Xie SY, Yen PM. Differential AMPK phosphorylation by glucagon and metformin regulates insulin signaling in human hepatic cells. Biochem Biophys Res Commun. 2014;447:569–73.
Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–51.
Burg N, Swendeman S, Worgall S, Hla T, Salmon JE. Sphingosine 1-phosphate receptor 1 signaling maintains endothelial cell barrier function and protects against immune complex-induced vascular injury. Arthritis Rheumatol. 2018;70:1879–89.
Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–94.
Chen X, Zhang C, Wang H, Xu J, Duan ZH, Zhang Y, et al. Altered integrity and decreased expression of hepatocyte tight junctions in rifampicin-induced cholestasis in mice. Toxicol Appl Pharmacol. 2009;240:26–36.
Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, et al. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29:469–81.
Dong J, Wang H, Zhao J, Sun J, Zhang T, Zuo L, et al. SEW2871 protects from experimental colitis through reduced epithelial cell apoptosis and improved barrier function in interleukin-10 gene-deficient mice. Immunol Res. 2015;61:303–11.
Dragsten PR, Handler JS, Blumenthal R. Fluorescent membrane probes and the mechanism of maintenance of cellular asymmetry in epithelia. Fed Proc. 1982;41:48–53.
Eloranta JJ, Kullak-Ublick GA. The role of FXR in disorders of bile acid homeostasis. Physiology (Bethesda). 2008;23:286–95.
Fu D, Wakabayashi Y, Ido Y, Lippincott-Schwartz J, Arias IM. Regulation of bile canalicular network formation and maintenance by AMP-activated protein kinase and LKB1. J Cell Sci. 2010;123:3294–302.
Fu D, Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proc Natl Acad Sci U S A. 2011;108:1403–8.
Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol. 2015;63:1023–37.
Halilbasic E, Claudel T, Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol. 2013;58:155–68.
Homolya L, Fu D, Sengupta P, Jarnik M, Gillet JP, Vitale-Cross L, et al. LKB1/AMPK and PKA control ABCB11 trafficking and polarization in hepatocytes. PLoS One. 2014;9:e91921.
Horikoshi Y, Kitatani K, Toriumi K, Fukunishi N, Itoh Y, Nakamura N, et al. Aberrant activation of atypical protein kinase C in carbon tetrachloride-induced oxidative stress provokes a disturbance of cell polarity and sealing of bile canalicular lumen. Am J Pathol. 2015;185:958–68.
Houten SM, Auwerx J. The enterohepatic nuclear receptors are major regulators of the enterohepatic circulation of bile salts. Ann Med. 2004;36:482–91.
Kawaguchi T, Sakisaka S, Sata M, Mori M, Tanikawa K. Different lobular distributions of altered hepatocyte tight junctions in rat models of intrahepatic and extrahepatic cholestasis. Hepatology. 1999;29:205–16.
Kleuser B. Divergent role of sphingosine 1-phosphate in liver health and disease. Int J Mol Sci. 2018;19:722.
Li Q, Chen B, Zeng C, Fan A, Yuan Y, Guo X, et al. Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells. Exp Physiol. 2015;100:95–107.
Li X, Liu R, Yu L, Yuan Z, Sun R, Yang H, et al. Alpha-naphthylisothiocyanate impairs bile acid homeostasis through AMPK-FXR pathways in rat primary hepatocytes. Toxicology. 2016a;370:106–15.
Li X, Yuan Z, Liu R, Hassan HM, Yang H, Sun R, et al. UDCA and CDCA alleviate 17α-ethinylestradiol-induced cholestasis through PKA-AMPK pathways in rats. Toxicol Appl Pharmacol. 2016b;15:12–25.
Li X, Liu R, Yang J, Sun L, Zhang L, Jiang Z, et al. The role of long noncoding RNA H19 in gender disparity of cholestatic liver injury in multidrug resistance 2 gene knockout mice. Hepatology. 2017a;66:869–84.
Li X, Liu R, Luo L, Yu L, Chen X, Sun L, et al. Role of AMP-activated protein kinase α1 in 17α-ethinylestradiol-induced cholestasis in rats. Arch Toxicol. 2017b;91:481–94.
Li X, Liu R, Zhang L, Jiang Z. The emerging role of AMP-activated protein kinase in cholestatic liver diseases. Pharmacol Res. 2017c;125:105–13.
Liang GH, Weber CR. Molecular aspects of tight junction barrier function. Curr Opin Pharmacol. 2014;19:84–9.
Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931.
Nho K, Kueider-Paisley A, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G, et al. Alzheimer's disease neuroimaging initiative and the Alzheimer disease metabolomics consortium. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: relationship to neuroimaging and CSF biomarkers. Alzheimers Dement. 2019;15:232–44.
Porat-Shliom N, Tietgens AJ, Van Itallie CM, Vitale-Cross L, Jarnik M, Harding OJ, et al. Liver kinase B1 regulates hepatocellular tight junction distribution and function in vivo. Version 2. Hepatology. 2016;64:1317–29.
Rida R, Kreydiyyeh S. FTY720P inhibits the Na+/K+ ATPase in Caco-2 cells via S1PR2: PGE2 and NO are along the signaling pathway. Life Sci. 2018;215:198–206.
Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–7.
Rowart P, Erpicum P, Krzesinski JM, Sebbagh M, Jouret F. Mesenchymal stromal cells accelerate epithelial tight junction assembly via the AMP-activated protein kinase pathway, independently of liver kinase B1. Stem Cells Int. 2017;2017:9717353.
Rowart P, Wu J, Caplan MJ, Jouret F. Implications of AMPK in the formation of epithelial tight junctions. Int J Mol Sci. 2018;13:2040.
Sakisaka S, Kawaguchi T, Taniguchi E, Hanada S, Sasatomi K, Koga H, et al. Alterations in tight junctions differ between primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2001;33:1460–8.
Sambrotta M, Strautnieks S, Papouli E, Rushton P, Clark BE. Parry DA, et al; mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326–8.
Santiago P, Scheinberg AR, Levy C. Cholestatic liver diseases: new targets, new therapies. Ther Adv Gastroenterol. 2018;11:1756284818787400.
Schlegel N, Waschke J. cAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier- a signaling pathway compromised in inflammation. Cell Tissue Res. 2014;355:587–96.
Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–35.
Stieger B, Landmann L. Effects of cholestasis on membrane flow and surface polarity in hepatocytes. J Hepatol. 1996;24:128–34.
Trauner M, Fuchs CD, Halilbasic E, Paumgartner G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology. 2017;65:1393–404.
Treyer A, Müsch A. Hepatocyte polarity. Compr Physiol. 2013;3:243–87.
Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;65:2005–18.
Xu XQ, Huang CM, Zhang YF, Chen L, Cheng H, Wang JM. S1PR1 mediates anti-apoptotic/pro-proliferative processes in human acute myeloid leukemia cells. Mol Med Rep. 2016;14:3369–75.
Yang T, Mei H, Xu D, Zhou W, Zhu X, Sun L, et al. Early indications of ANIT-induced cholestatic liver injury: alteration of hepatocyte polarization and bile acid homeostasis. Food Chem Toxicol. 2017a;110:1–12.
Yang T, Shu T, Liu G, Mei H, Zhu X, Huang X, et al. Quantitative profiling of 19 bile acids in rat plasma, liver, bile and different intestinal section contents to investigate bile acid homeostasis and the application of temporal variation of endogenous bile acids. J Steroid Biochem Mol Biol. 2017b;172:69–78.
Yang T, Khan GJ, Wu Z, Wang X, Zhang L, Jiang Z. Bile acid homeostasis paradigm and its connotation with cholestatic liver diseases. Drug Discov Today. 2019;24:112–28.
Yang T, Wang X, Yuan Z, Miao Y, Wu Z, Chai Y, et al. Sphingosine 1-phosphate receptor-1 specific agonist SEW2871 ameliorates ANIT-induced dysregulation of bile acid homeostasis in mice plasma and liver. Toxicol Lett. 2020;331:242–53.
Yu L, Liu X, Yuan Z, Li X, Yang H, Yuan Z, et al. SRT1720 alleviates ANIT-induced cholestasis in a mouse model. Front Pharmacol. 2017;8:256.
Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastrointestinal and liver disease. Gut. 2019;68:547–61.
Author information
Authors and Affiliations
Contributions
Zhenzhou Jiang, Luyong Zhang, and Tingting Yang conceived the original ideas and designed the study. Tingting Yang did immunofluorescence, hierarchical clustered heat map analysis and PCA analysis. Xue Wang carried out the HPLC-MS/MS analysis, quantitative real-time-PCR, and measurement of inflammatory cytokines. Yi Zhou and Cai Heng helped by doing MPO activity analysis and western blot analysis of animals and cells. Qiongna Yu carried out the isolation and sandwich culture of mice primary hepatocyte. Hao Yang did hematoxylin-eosin staining and immunohistochemistry. Tingting Yang, Xue Wang, Yi Zhou, and Cai Heng analyzed the data. Tingting Yang and Hao Yang edited of the figures and tables. Tingting Yang and Zhenzhou Jiang wrote the manuscript. Zhenzhou Jiang, Luyong Zhang, and Tingting Yang reviewed the manuscript. The rest of the authors either supplied reagent or helped in treatments to mice and cells.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 86.1 kb).
Rights and permissions
About this article
Cite this article
Yang, T., Wang, X., Zhou, Y. et al. SEW2871 attenuates ANIT-induced hepatotoxicity by protecting liver barrier function via sphingosine 1-phosphate receptor-1–mediated AMPK signaling pathway. Cell Biol Toxicol 37, 595–609 (2021). https://doi.org/10.1007/s10565-020-09567-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-020-09567-9