Abstract
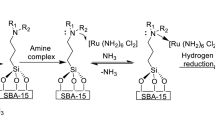
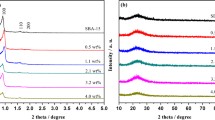
The gradual rise in CO2 emission urged the search for different techniques not only to capture the CO2 gas but also transform the same in useful chemicals such as formic acid. In the present study, we established an effective protocol to support Ru NPs on amine-functionalized SBA-15 mesoporous silica and further utilized them as catalysts for the direct hydrogenation of CO2 gas into the formic acid. The presence of organoamine groups around the Ru NPs plays a significant role to stabilize the Ru NPs followed by strong metal-molecular support interaction (between Ru NPs and SBA-15) and delivering the improved catalytic activities for the easy CO2 reduction into the formic acid formation. We reported a comprehensive study on the impacts of various amine groups on the catalytic performance of amine-functionalized SBA-15 supported Ru catalysts for formic acid formation. We also demonstrated a comprehensive study of different amine groups on the catalytic performance of ultrafine uniformly dispersed Ru NPs over mesoporous SBA-15 support. The effect of various compositional and steric properties of amine groups on the size/distribution of the Ru NPs were closely studied and correlated with their catalytic performance in the CO2 hydrogenation reaction. We successfully recycled the catalysts up to five runs with good catalytic activity.










Similar content being viewed by others
References
Styring P, Quadrelli EA, Armstrong K (2014) Carbon dioxide utilisation: closing the carbon cycle, 1st edn. Elsevier, Inc., Amsterdam
Leung DYC, Caramanna G, Maroto-Valer MM (2014) An overview of current status of carbon dioxide capture and storage technologies. Renew Sustain Energy Rev 39:426–443
Aresta M (1999) Perspectives in the use of carbon dioxide. Quim Nova 22:269–272
de Arajo OQ, de Medeiros JL, Maria R (2014) CO2 utilization: a process systems engineering vision. In: CO2 sequestration and valorization. InTech, Rijeka
Liu Q, Wu L, Jackstell R, Beller M (2015) Using carbon dioxide as a building block in organic synthesis. Nat Commun 6:1–15
Artz J, Müller TE, Thenert K et al (2018) Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem Rev 118:434–504
Sakakura T, Choi JC, Yasuda H (2007) Transformation of carbon dioxide. Chem Rev 107:2365–2387
Hietala J, Vuori A, Johnsson P et al (2016) Formic acid. In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1–22
Mellmann D, Sponholz P, Junge H, Beller M (2016) Formic acid as a hydrogen storage material-development of homogeneous catalysts for selective hydrogen release. Chem Soc Rev 45:3954–3988
Singh AK, Singh S, Kumar A (2016) Hydrogen energy future with formic acid: a renewable chemical hydrogen storage system. Catal Sci Technol 6:12–40
Centi G, Quadrelli EA, Perathoner S (2013) Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ Sci 6:1711–1731
Pérez-Fortes M, Schöneberger JC, Boulamanti A et al (2016) Formic acid synthesis using CO2 as raw material: techno-economic and environmental evaluation and market potential. Int J Hydrog Energy 41:16444–16462. https://doi.org/10.1016/j.ijhydene.2016.05.199
Jo DY, Ham HC, Lee KY (2020) Facet-dependent electrocatalysis in the HCOOH synthesis from CO2 reduction on Cu catalyst: a density functional theory study. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2020.146857
Zhang Y, Zhang T, Das S (2020) Catalytic transformation of CO2 into C1 chemicals using hydrosilanes as a reducing agent. Green Chem 22:1800–1820
Leitner W (1995) Carbon dioxide as a raw material: the synthesis of formic acid and its derivatives from CO2. Angew Chem Int Ed Engl 34:2207–2221
Reymond H, Corral-Pérez JJ, Urakawa A, Von RudolfRohr P (2018) Towards a continuous formic acid synthesis: a two-step carbon dioxide hydrogenation in flow. React Chem Eng 3:912–919. https://doi.org/10.1039/c8re00142a
Jessop PG, Ikariya T, Noyori R (1995) Homogeneous hydrogenation of carbon dioxide. Chem Rev 95:259–272. https://doi.org/10.1021/cr00034a001
Wang W-H, Feng X, Bao M (2018) Transformation of carbon dioxide to formic acid and methanol. Springer, Singapore
Wang W-H, Hime Y (2012) Recent advances in transition metal-catalysed homogeneous hydrogenation of carbon dioxide in aqueous media. In: Hydrogenation. InTech, Rijeka
Jessop PG, Ikariya T, Noyori R (1994) Homogeneous catalytic hydrogenation of supercritical carbon dioxide. Nature 368:231–233. https://doi.org/10.1038/368231a0
Wang W-H, Feng X, Bao M (2018) Transformation of CO2 to formic acid or formate with homogeneous catalysts. Springer, Singapore, pp 7–42
Jessop PG, Joó F, Tai CC (2004) Recent advances in the homogeneous hydrogenation of carbon dioxide. Coord Chem Rev 248:2425–2442
Ross JRH (2012) Heterogeneous catalysis—chemistry in two dimensions. In: Heterogeneous catalysis. Elsevier, Amsterdam, pp 1–15
Gunasekar GH, Park K, Jung KD, Yoon S (2016) Recent developments in the catalytic hydrogenation of CO2 to formic acid/formate using heterogeneous catalysts. Inorg Chem Front 3:882–895
Bulushev DA, Ross JRH (2018) Heterogeneous catalysts for hydrogenation of CO2 and bicarbonates to formic acid and formates. Catal Rev Sci Eng 60:566–593. https://doi.org/10.1080/01614940.2018.1476806
Upadhyay PR, Srivastava V (2017) Ionic liquid mediated in situ synthesis of Ru nanoparticles for CO2 hydrogenation reaction. Catal Lett 147:1051–1060. https://doi.org/10.1007/s10562-017-1995-7
Gautam P, Upadhyay PR, Srivastava V (2019) Selective hydrogenation of CO2 to formic acid over alumina-supported Ru nanoparticles with multifunctional ionic liquid. Catal Lett 149:1464–1475. https://doi.org/10.1007/s10562-019-02773-z
Liu Q, Yang X, Li L et al (2017) Direct catalytic hydrogenation of CO2 to formate over a Schiff-base-mediated gold nanocatalyst. Nat Commun 8:1–8. https://doi.org/10.1038/s41467-017-01673-3
Masuda S, Mori K, Kuwahara Y et al (2020) Additive-free aqueous phase synthesis of formic acid by direct CO2 hydrogenation over a PdAg catalyst on a hydrophilic N-doped polymer-silica composite support with high CO2 affinity. ACS Appl Energy Mater 3:5847–5855. https://doi.org/10.1021/acsaem.0c00771
Wang W, Wang S, Ma X, Gong J (2011) Recent advances in catalytic hydrogenation of carbon dioxide. Chem Soc Rev 40:3703–3727. https://doi.org/10.1039/c1cs15008a
Xi J, Wang Q, Duan X et al (2020) Continuous flow reduction of organic dyes over Pd–Fe alloy based fibrous catalyst in a fixed-bed system. Chem Eng Sci 231:116303. https://doi.org/10.1016/j.ces.2020.116303
Martín-Jimeno FJ, Suárez-García F, Paredes JI et al (2021) Nickel nanoparticle/carbon catalysts derived from a novel aqueous-synthesized metal–organic framework for nitroarene reduction. J Alloys Compd 853:157348. https://doi.org/10.1016/j.jallcom.2020.157348
Liu J, Hao J, Hu C et al (2018) Palladium nanoparticles anchored on amine-functionalized silica nanotubes as a highly effective catalyst. J Phys Chem C 122:2696–2703. https://doi.org/10.1021/acs.jpcc.7b10237
Srivastava V (2016) Active heterogeneous Ru nanocatalysts for CO2 hydrogenation reaction. Catal Lett 146:2630–2640. https://doi.org/10.1007/s10562-016-1882-7
Srivastava V (2014) Ru-exchanged MMT clay with functionalized ionic liquid for selective hydrogenation of CO2 to formic acid. Catal Lett 144:2221–2226. https://doi.org/10.1007/s10562-014-1392-4
Fan J, Gao Y (2006) Nanoparticle-supported catalysts and catalytic reactions—a mini-review. J Exp Nanosci 1:457–475. https://doi.org/10.1080/17458080601067708
Kokunešoski M, Gulicovski J, Matović B et al (2010) Synthesis and surface characterization of ordered mesoporous silica SBA-15. Mater Chem Phys 124:1248–1252. https://doi.org/10.1016/j.matchemphys.2010.08.066
Allen AD, Senoff CV (1967) Preparation and infrared spectra of some ammine complexes of ruthenium(II) and ruthenium(III). Can J Chem 45:1337–1341. https://doi.org/10.1139/v67-220
Zhao D, Feng J, Huo Q et al (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 Angstrom pores. Science 279:548–552. https://doi.org/10.1126/science.279.5350.548
Gu D, Schüth F (2014) Synthesis of non-siliceous mesoporous oxides. Chem Soc Rev 43:313–344
Hsu YC, Hsu YT, Hsu HY, Yang CM (2007) Facile synthesis of mesoporous silica SBA-15 with additional intra-particle porosities. Chem Mater 19:1120–1126. https://doi.org/10.1021/cm062167i
Koh K, Jeon M, Yoon CW, Asefa T (2017) Formic acid dehydrogenation over Pd NPs supported on amine-functionalized SBA-15 catalysts: structure–activity relationships. J Mater Chem A 5:16150–16161. https://doi.org/10.1039/c7ta02040f
Jiang T, Ma X, Zhou Y et al (2008) Solvent-free synthesis of substituted ureas from CO2 and amines with a functional ionic liquid as the catalyst. Green Chem 10:465–469. https://doi.org/10.1039/b717868a
Wang L, Yang RT (2011) Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density. J Phys Chem C 115:21264–21272. https://doi.org/10.1021/jp206976d
Park S, Choi K, Yu HJ et al (2018) Thermal stability enhanced tetraethylenepentamine/silica adsorbents for high performance CO2 capture. Ind Eng Chem Res 57:4632–4639. https://doi.org/10.1021/acs.iecr.7b04912
Jo SB, Chae HJ, Kim TY et al (2020) Thermally stable amine-functionalized silica sorbents using one-pot synthesis method for CO2 capture at low temperature. Korean J Chem Eng 37:2317–2325. https://doi.org/10.1007/s11814-020-0655-6
Qadir K, Kim SM, Seo H et al (2013) Deactivation of Ru catalysts under catalytic CO oxidation by formation of bulk Ru oxide probed with ambient pressure XPS. J Phys Chem C 117:13108–13113. https://doi.org/10.1021/jp402688a
Carbonio EA, Prieto MJ, De Siervo A, Landers R (2014) From 1D to 3D Ru nanostructures on a pt stepped surface as model systems in electrocatalysis: UHV-STM and XPS study. J Phys Chem C 118:28679–28688. https://doi.org/10.1021/jp509574s
Qadir K, Joo SH, Mun BS et al (2012) Intrinsic relation between catalytic activity of CO oxidation on Ru nanoparticles and Ru oxides uncovered with ambient pressure XPS. Nano Lett 12:5761–5768. https://doi.org/10.1021/nl303072d
Upadhyay PR, Srivastava V (2016) Selective hydrogenation of CO2 gas to formic acid over nanostructured Ru–TiO2 catalysts. RSC Adv 6:42297–42306. https://doi.org/10.1039/c6ra03660k
Srivastava V (2018) Functionalized hydrotalcite tethered ruthenium catalyst for carbon sequestration reaction. Catal Lett 148:1879–1892. https://doi.org/10.1007/s10562-018-2399-z
Chiang CL, Lin KS, Chuang HW, Wu CM (2017) Conversion of hydrogen/carbon dioxide into formic acid and methanol over Cu/CuCr2O4 catalyst. Int J Hydrog Energy 42:23647–23663. https://doi.org/10.1016/j.ijhydene.2017.04.226
Hiyoshi N, Yogo K, Yashima T (2005) Adsorption of carbon dioxide on aminosilane-modified mesoporous silica. J Jpn Pet Inst 48:29–36. https://doi.org/10.1627/jpi.48.29
Pandey PH, Pawar HS (2020) Cu dispersed TiO2 catalyst for direct hydrogenation of carbon dioxide into formic acid. J CO2 Util 41:101267. https://doi.org/10.1016/j.jcou.2020.101267
Gautam P, Srivastava V (2020) Active γ-alumina-supported Ru nanoparticles for TiO2 hydrogenation reaction. Lett Org Chem 17:603–612. https://doi.org/10.2174/1570178617666191107112429
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Srivastava, V. Amine‐Functionalized SBA-15 Supported Ru Nanocatalyst for the Hydrogenation CO2 to Formic Acid. Catal Surv Asia 25, 192–205 (2021). https://doi.org/10.1007/s10563-021-09325-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-021-09325-9