Abstract
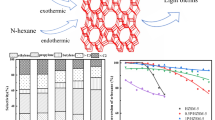
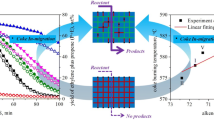
The deactivation mechanism of HZSM-5 zeolite in the co-cracking of n-hexane and alcohols was studied. The fresh and spent catalysts were characterized by X-ray diffraction (XRD), N2 adsorption-desorption isotherms, NH3-TPD, gas chromatography-mass spectrometry (GC-MS), high-resolution time-of-flight mass spectrometry (ESI-TOF), thermogravimetric analyzer (TGA), Fourier infrared spectroscopy (FT-IR), 13C MAS NMR, and H2-TPR. The results showed that the internal coke is preferentially formed in the zeolite micropores in the initial reaction stage, leading to the partial deactivation of catalyst. The addition of alcohols to n-hexane significantly promoted the formation of external coke, which hardly had effect on the cracking activity. H2 treatment to the spent catalyst at 800 °C could effectively remove the internal coke of the catalyst, while the external coke with a graphite-like structure still kept stable. Methylated aromatics were considered coke precursors. It was reasonably proposed that methylated aromatics condense to polyaromatic structures as internal coke in the presence of acid sites, but their growth is limited by the catalyst channel structure. This work demonstrated that zeolite deactivation behavior was affected by the location, amount, and type of coke. The formation mechanism of internal and external coke as well as the H2 decoking to generate deactivated catalyst were discussed.
Graphical Abstract






















Similar content being viewed by others
References
Gholami Z, Gholami F, Tišler Z, Tomas M, Vakili M (2021) A review on production of light olefins via fluid catalytic cracking. Energies 14:1089
Sadrameli SM (2016) Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: a state-of-the-art review II: Catalytic cracking review. Fuel 173:285–297
Fu T, Guo Y, Li Z, Zhan G (2022) Selective conversion of methanol to aromatics with superior catalytic stability by relay catalysis over quadruple ZSM-5 sequence beds with gradient-increasing acidity. Fuel 315:123241
Chang F, Wei Y, Liu X, Qi Y, Zhang D, He Y, Liu Z (2006) An improved catalytic cracking of n-hexane via methanol coupling reaction over HZSM-5 zeolite catalysts. Catal Lett 106:171–176
Cheng Q, Shen B, Liu J, Qin X, Ye L, Xing B (2021) Reaction kinetic model of naphtha–methanol catalytic conversion for light olefins over HZSM-5 based on structure-oriented lumping. Energy Fuels 35:10786–10795
Chang F, Wei Y, Liu X, Zhao Y, Xu L, Sun Y, Zhang D, He Y, Liu Z (2007) A mechanistic investigation of the coupled reaction of n-hexane and methanol over HZSM-5. Appl Catal A 328:163–173
Zhu X, Wang Y, Wang G, Hou Y, Yu M, Yang X, Yin F (2021) Synergistic co-conversion of pentane and methanol to aromatics over bifunctional metal/ZSM-5 zeolite catalysts. Microporous Mesoporous Mater 320:111107
Ilias S, Bhan A (2012) Mechanism of the catalytic conversion of methanol to hydrocarbons. ACS Catal 3:18–31
Fakhroleslam M, Sadrameli SM (2019) Thermal/catalytic cracking of hydrocarbons for the production of olefins; a state-of-the-art review III: Process modeling and simulation. Fuel 252:553–566
Shen K, Wang N, Qian W, Cui Y, Wei F (2014) Atmospheric pressure synthesis of nanosized ZSM-5 with enhanced catalytic performance for methanol to aromatics reaction. Catal Sci Technol 4:3840–3844
He M, Ali M-F, Song Y-Q, Zhou X-L, Wang JA, Nie X-Y, Wang Z (2023) Study on the deactivation mechanism of HZSM-5 in the process of catalytic cracking of n-hexane. Chem Eng J 451:138793
Zhang H, Shao S, Xiao R, Shen D, Zeng J (2013) Characterization of coke deposition in the catalytic fast pyrolysis of biomass derivates. Energy Fuels 28:52–57
Castaño P, Elordi G, Ibañez M, Olazar M, Bilbao J (2012) Pathways of coke formation on an MFI catalyst during the cracking of waste polyolefins. Catal Sci Technol 2:504–508
Nakasaka Y, Nishimura J-I, Tago T, Masuda T (2015) Deactivation mechanism of MFI-type zeolites by coke formation during n-hexane cracking. Chem Eng J 278:159–165
Cordero-Lanzac T, Ateka A, Pérez-Uriarte P, Castaño P, Aguayo AT, Bilbao J (2018) Insight into the deactivation and regeneration of HZSM-5 zeolite catalysts in the conversion of dimethyl ether to olefins. Ind Eng Chem Res 57:13689–13702
An H, Zhang F, Guan Z, Liu X, Fan F, Li C (2018) Investigating the coke formation mechanism of H-ZSM-5 during methanol dehydration using operando UV-Raman spectroscopy. ACS Catal 8:9207–9215
Li X-G, Huang X, Zhang Y-L, Li H, Xiao W-D, Wei Z (2020) Effect of n-butanol cofeeding on the deactivation of methanol to olefin conversion over high-silica HZSM-5: a mechanism and kinetic study. Chem Eng Sci 226:115859
Mores D, Stavitski E, Kox MH, Kornatowski J, Olsbye U, Weckhuysen BM (2008) Space- and time-resolved in-situ spectroscopy on the coke formation in molecular sieves: methanol-to-olefin conversion over H-ZSM-5 and H-SAPO-34. Chemistry 14:11320–11327
Urata K, Furukawa S, Komatsu T (2014) Location of coke on H-ZSM-5 zeolite formed in the cracking of n-hexane. Appl Catal A 475:335–340
Lee S, Choi M (2019) Unveiling coke formation mechanism in MFI zeolites during methanol-to-hydrocarbons conversion. J Catal 375:183–192
Paunović V, Hemberger P, Bodi A, Hauert R, van Bokhoven JA (2022) Impact of nonzeolite-catalyzed formation of formaldehyde on the methanol-to-hydrocarbons conversion. ACS Catal 12:13426–13434
Guisnet M, Costa L, Ribeiro FR (2009) Prevention of zeolite deactivation by coking. J Mol Catal A Chem 305:69–83
Wang Z, Zhang R, Wang J, Yu Z, Xiang Y, Kong L, Liu H, Ma A (2022) Hierarchical zeolites obtained by alkaline treatment for enhanced n-pentane catalytic cracking. Fuel 313:122669
Hou X, Qiu Y, Tian Y, Diao Z, Zhang X, Liu G (2018) Reaction pathways of n-pentane cracking on the fresh and regenerated Sr, Zr and La-loaded ZSM-5 zeolites. Chem Eng J 349:297–308
Wei S, Xu Y, Jin Z, Zhu X (2020) Co-conversion of methanol and n-hexane into aromatics using intergrown ZSM-5/ZSM-11 as a catalyst. Front Chem Sci Eng 14:783–792
Lian Z, Si C, Jan F, Zhi S, Li B (2021) Coke deposition on Pt-based catalysts in propane direct dehydrogenation: kinetics, suppression, and elimination. ACS Catal 11:9279–9292
Gounder R, Iglesia E (2011) Catalytic hydrogenation of alkenes on acidic zeolites: mechanistic connections to monomolecular alkane dehydrogenation reactions. J Catal 277:36–45
Alvarez AG, Viturro H, Bonetto RD (1992) Structural changes on deactivation of ZSM-5. A study by X-ray powder diffraction. Mater Chem Phys 32:135–140
Rojo-Gama D, Nielsen M, Wragg DS, Dyballa M, Holzinger J, Falsig H, Lundegaard LF, Beato P, Brogaard RY, Lillerud KP, Olsbye U, Svelle S (2017) A straightforward descriptor for the deactivation of zeolite catalyst H-ZSM-5. ACS Catal 7:8235–8246
Hou X, Qiu Y, Zhang X, Liu G (2017) Effects of regeneration of ZSM-5 based catalysts on light olefins production in n-pentane catalytic cracking. Chem Eng J 321:572–583
Meinhold RH, Bibby DM (1990) 27AI and 29Si NMR, studies of HZSM-5: Part 2 The effect of coke formation. Zeolites 10:146–150
Barbera K, Sørensen S, Bordiga S, Skibsted J, Fordsmand H, Beato P, Janssens TVW (2012) Role of internal coke for deactivation of ZSM-5 catalysts after low temperature removal of coke with NO2. Catal Sci Technol 2:1196–1206
Bonardet JL, Barrage MC, Fraissard J (1995) Use of NMR techniques for studying deactivation of zeolites by coking. J Mol Catal A Chem 96:123–143
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, M., Qie, G., Ali, M.F. et al. Study of Coke Formation Mechanism on HZSM-5 Zeolite During Co-cracking of n-Hexane and Alcohols. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04583-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04583-4