Abstract
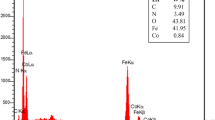
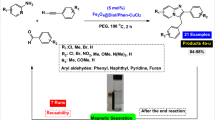
In recent times, the research on the synthesis of highly functionalized piperidines has become a challenge and an attractive research field among synthetic chemists because these compounds have many biological, pharmaceutical and industrial applications. In this regard, in this paper we would like to report a green, rapid and efficient method for the synthesis of highly functionalized piperidines based on the use of zinc (II) chloride supported on Fe3O4 NPs modified with Dopamine and 5H-Cyclopenta[2,1-b:3,4-b′]dipyridin-5-one [Fe3O4@Dop/CP-diPy-ZnCl2 as a novel magnetically reusable catalyst. The structure of Fe3O4@Dop/CP-diPy-ZnCl2 nanocatalyst was well identified by several spectroscopic techniques including: FT-IR, TEM, SEM, EDX, ICP-OES, TGA, XRD, VSM and Elemental mapping technique. One-pot three-component reactions of aromatic amines with different aryl aldehydes and β-ketoesters were performed in water and all desired highly functionalized piperidines were synthesized with high to excellent yields, which indicates the high efficiency of this catalytic system. The reusability tests shown that that Fe3O4@Dop/CP-diPy-ZnCl2 catalyst is a reusable and stable catalyst.
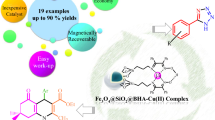
Graphical Abstract

















Similar content being viewed by others
References
Das S, Santra S, Roy A et al (2012) One-pot multicomponent synthesis of polyhydroquinolines under catalyst and solvent-free conditions. Green Chem Lett Rev 5:97–100. https://doi.org/10.1080/17518253.2011.584073
Kazemi M (2023) Copper catalysts immobilized on magnetic nanoparticles: catalysis in synthesis of tetrazoles. Nanomater Chem 1:1–11. https://doi.org/10.22034/nc.2023.416867.1003
Khan AT, Khan MM, Bannuru KKR (2010) Iodine catalyzed one-pot five-component reactions for direct synthesis of densely functionalized piperidines. Tetrahedron 66:7762–7772. https://doi.org/10.1016/j.tet.2010.07.075
NooryFajer A, KhabtAboud H, Al-Bahrani HA, Kazemi M (2023) Recent advances on multicomponent synthesis of pyranopyrazoles using magnetically recoverable nanocatalysts. Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2023.2255723
Bhat AR, Shalla AH, Dongre RS (2017) Synthesis of new annulated pyrano[2,3-d]pyrimidine derivatives using organo catalyst (DABCO) in aqueous media. J Saudi Chem Soc 21:S305–S310. https://doi.org/10.1016/j.jscs.2014.03.008
Wan Q, Huang CY, Hou ZW et al (2023) Organophotoelectrochemical silylation cyclization for the synthesis of silylated 3-CF 3–2-oxindoles. Org Chem Front. https://doi.org/10.1039/D3QO00728F
Chen D, Wang Q, Li Y et al (2020) A general linear free energy relationship for predicting partition coefficients of neutral organic compounds. Chemosphere 247:125869. https://doi.org/10.1016/j.chemosphere.2020.125869
He B, Hou F, Ren C et al (2021) A review of current in silico methods for repositioning drugs and chemical compounds. Front Oncol. https://doi.org/10.3389/fonc.2021.711225
Kazemi M, Karezani N (2023) Research on biological and bioactive molecules containing pyrrole scaffolds. Biol Mol Chem 1:15–26. https://doi.org/10.22034/bmc.2023.414945.1003
Zhang Y, Song N (2023) Co3O4 nanocatalyst: a useful heterogeneous catalyst for the synthesis of chloroquine in the treatment of malaria. Biol Mol Chem 1:53–60. https://doi.org/10.22034/bmc.2023.418539.1008
Chen Y, Zhang Z, Jiang W et al (2019) RuIII@CMC/Fe3O4 hybrid: an efficient, magnetic, retrievable, self-organized nanocatalyst for green synthesis of pyranopyrazole and polyhydroquinoline derivatives. Mol Divers 23:421–442. https://doi.org/10.1007/s11030-018-9887-3
Moghadasi Z, NooryFajer A, Abudken MHA, Ali Al-Bahrani H (2023) One-pot three-component synthesis of 2-substituted benzothiazoles using Fe3O4 nanoparticles modified with serine supported Cu (I) iodide. Nanomater Chem 1:24–31. https://doi.org/10.22034/nc.2023.419246.1006
Zhang Z, Zhang W, Hou ZW et al (2023) Electrophilic halospirocyclization of N-benzylacrylamides to access 4-halomethyl-2-azaspiro[4.5]decanes. J Org Chem 88:13610–13621. https://doi.org/10.1021/acs.joc.3c01315
Dadaei M, Naeimi H (2021) Nano cobalt ferrite encapsulated-silica particles bearing melamine as an easily recyclable catalyst for the synthesis of dihydropyrano[2,3- c ]pyrazoles under green conditions. Appl Organomet Chem. https://doi.org/10.1002/aoc.6365
Mishra S, Karabiyikoglu S, Fletcher SP (2023) Catalytic enantioselective synthesis of 3-piperidines from arylboronic acids and pyridine. J Am Chem Soc 145:14221–14226. https://doi.org/10.1021/jacs.3c05044
Frolov NA, Vereshchagin AN (2023) Piperidine derivatives: recent advances in synthesis and pharmacological applications. Int J Mol Sci 24:2937. https://doi.org/10.3390/ijms24032937
Nairoukh Z, Wollenburg M, Schlepphorst C et al (2019) The formation of all-cis-(multi)fluorinated piperidines by a dearomatization–hydrogenation process. Nat Chem 11:264–270. https://doi.org/10.1038/s41557-018-0197-2
Paudel S, Wang S, Kim E et al (2022) Design, synthesis, and functional evaluation of 1, 5-disubstituted tetrazoles as monoamine neurotransmitter reuptake inhibitors. Biomol Ther 30:191–202. https://doi.org/10.4062/BIOMOLTHER.2021.119
Aboonajmi J, Mousavi MR, Maghsoodlou MT et al (2015) ZrCl4 as an efficient catalyst for one-pot synthesis of highly functionalized piperidines via multi-component organic reactions. Res Chem Intermed 41:1925–1934. https://doi.org/10.1007/s11164-013-1320-z
Agrawal NR, Bahekar SP, Sarode PB et al (2015) L-proline nitrate: a recyclable and green catalyst for the synthesis of highly functionalized piperidines. RSC Adv 5:47053–47059. https://doi.org/10.1039/c5ra08022c
GhamariKargar P, Bagherzade G (2021) Robust, highly active, and stable supported Co(ii) nanoparticles on magnetic cellulose nanofiber-functionalized for the multi-component reactions of piperidines and alcohol oxidation. RSC Adv 11:23192–23206. https://doi.org/10.1039/d1ra00208b
Sajadikhah SS, Hazeri N, Maghsoodlou MT et al (2012) One-pot three-component synthesis of highly substituted piperidines using 1-methyl-2-oxopyrrolidinium hydrogen sulfate. J Chem Res 36:463–467. https://doi.org/10.3184/174751912X13395258340271
Yan L, Li Y, Yang B, Gao W (2022) InBr 3-catalyzed synthesis of highly functionalized piperidines and benzo[a]pyrano[2,3-c] phenazines. Polycycl Aromat Compd 42:534–542. https://doi.org/10.1080/10406638.2020.1744026
Luo N, Wang S, Zhang Y et al (2020) DBU-promoted cascade selective nucleophilic addition/c-c bond cleavage/hetero-diels-alder reactions of 2-amino-4 h-chromen-4-ones with β-nitrostyrenes and/or aryl aldehydes: access to 5 h-chromeno[2,3- b]pyridin-5-ones. J Org Chem 85:14219–14228. https://doi.org/10.1021/acs.joc.0c01993
Hazeri N, Fereidooni E (2015) Trichloroacetic acid as an efficient catalyst for one-pot synthesis of highly functionalized piperidines via multi-component reaction. J Appl Chem Res 9:81–89
Khan AT, Parvin T, Choudhury LH (2008) Effects of substituents in the β-position of 1,3-dicarbonyl compounds in bromodimethylsulfonium bromide-catalyzed multicomponent reactions: a facile access to functionalized piperidines. J Org Chem 73:8398–8402. https://doi.org/10.1021/jo8014962
Ardakani LS, Arabmarkadeh A, Kazemi M (2021) Multicomponent synthesis of highly functionalized piperidines. Synth Commun 51:856–879. https://doi.org/10.1080/00397911.2020.1861301
Khan AT, Lal M, Khan MM (2010) Synthesis of highly functionalized piperidines by one-pot multicomponent reaction using tetrabutylammonium tribromide (TBATB). Tetrahedron Lett 51:4419–4424. https://doi.org/10.1016/j.tetlet.2010.06.069
Mousavi MR, Aboonajmi J, Maghsoodlou MT et al (2013) ChemInform abstract: La(NO 3) 3 ·6H 2 O catalyzed one-pot highly diastereoselective synthesis of functionalized piperidines. ChemInform. https://doi.org/10.1002/chin.201340152
Shen J, Gao Q, Wang G et al (2019) Cu-NHC-catalyzed enantioselective conjugate silyl addition to indol-1-ylacrylate derivatives. ChemistrySelect 4:11358–11361. https://doi.org/10.1002/slct.201903570
Arabmarkadeh A, Javahershenas R, Kazemi M (2021) Nanomaterials: catalysis in synthesis of highly substituted heterocycles. Synth Commun 51:880–903. https://doi.org/10.1080/00397911.2020.1864646
Yang S, Wu C, Zhou H et al (2013) An Ullmann C–O coupling reaction catalyzed by magnetic copper ferrite nanoparticles. Adv Synth Catal 355:53–58. https://doi.org/10.1002/adsc.201200600
Jdanova S, Taylor MS (2023) Mechanistic study of the copper(II)-mediated site-selective O -arylation of glycosides with arylboronic acids. J Org Chem 88:3487–3498. https://doi.org/10.1021/acs.joc.2c02693
Rafiee T, Zolfaghari Z (2022) Preparation and study of surface modified ZnO nanoparticles in copoly (amid-imide) nanocomposite films containing triptycene. J Synth Chem 1:108–115. https://doi.org/10.22034/jsc.2022.155237
Nakhate AV, Yadav GD (2017) Solvothermal synthesis of CuFe 2 O 4 @rGO: efficient catalyst for C-O cross coupling and N- arylation reaction under ligand-free condition. ChemistrySelect 2:7150–7159. https://doi.org/10.1002/slct.201700556
Cheng Q, Cao G, Bai Y et al (2023) Probing the demulsification mechanism of emulsion with SPAN series based on the effect of solid phase particles. Molecules 28:3261. https://doi.org/10.3390/molecules28073261
Kazemi N, MahdaviShahri M (2017) Magnetically separable and reusable CuFe2O4 spinel nanocatalyst for the O-arylation of phenol with aryl halide under ligand-free condition. J Inorg Organomet Polym Mater 27:1264–1273. https://doi.org/10.1007/s10904-017-0574-0
Li H, Wang Y, Jiang F et al (2023) A dual-function [Ru(bpy) 3 ] 2+ encapsulated metal organic framework for ratiometric Al 3+ detection and anticounterfeiting application. Dalt Trans 52:3846–3854. https://doi.org/10.1039/D2DT03388G
Paul S, Pradhan K, Ghosh S et al (2014) Magnetically retrievable nano crystalline nickel ferrite-catalyzed aerobic, ligand-free C–N, C–O and C–C cross-coupling reactions for the synthesis of a diversified library of heterocyclic molecules. Adv Synth Catal 356:1301–1316. https://doi.org/10.1002/adsc.201300686
Gong X, Shen Z, Wang G et al (2021) Heterogeneous copper-catalyzed synthesis of diaryl sulfones. Org Biomol Chem 19:10662–10668. https://doi.org/10.1039/D1OB01830B
Ghobakhloo F, Azarifar D, Mohammadi M et al (2022) Copper(II) Schiff-base complex modified UiO-66-NH2(Zr) metal-organic framework catalysts for knoevenagel condensation-michael addition-cyclization reactions. Inorg Chem 61:4825–4841. https://doi.org/10.1021/acs.inorgchem.1c03284
Fajer AN, Al-Bahrani HA, Kadhum AAH, Kazemi M (2024) Research on catalytic application of Fe3O4@AAPA-AP-CuCl2 nanocomposite: first nanomagnetic copper catalyst for preparation of aryl nitriles from aldehydes. J Mol Struct 1296:136800. https://doi.org/10.1016/j.molstruc.2023.136800
Kazemi M (2020) Based on magnetic nanoparticles: gold reusable nanomagnetic catalysts in organic synthesis. Synth Commun 50:2079–2094. https://doi.org/10.1080/00397911.2020.1725058
Sun L, Liang T, Zhang C, Chen J (2023) The rheological performance of shear-thickening fluids based on carbon fiber and silica nanocomposite. Phys Fluids. https://doi.org/10.1063/5.0138294
Ghobadi M (2022) Based on copper ferrite nanoparticles (CuFe2O4 NPs): catalysis in synthesis of heterocycles. J Synth Chem 1:84–96. https://doi.org/10.22034/jsc.2022.155234
Shinde G, Thakur J (2022) Magnetically recyclable Ag@Fe2O3 Core-shell nanostructured catalyst for one-pot synthesis of 2-aryl benzimidazole and benzothiazole. Curr Organocatal 9:237–251. https://doi.org/10.2174/2213337209666220329125047
Rostami A, Pourshiani O, Navasi Y et al (2017) Magnetic nanoparticle-supported DABCO tribromide: a versatile nanocatalyst for the synthesis of quinazolinones and benzimidazoles and protection/deprotection of hydroxyl groups. New J Chem 41:9033–9040. https://doi.org/10.1039/C7NJ00479F
Mohammadi M, Khodamorady M, Tahmasbi B et al (2021) Boehmite nanoparticles as versatile support for organic–inorganic hybrid materials: Synthesis, functionalization, and applications in eco-friendly catalysis. J Ind Eng Chem 97:1–78. https://doi.org/10.1016/j.jiec.2021.02.001
Shamaei A, Mahmoudi B, Kazemnejadi M, Nasseri MA (2020) Mg-catalyzed one-pot preparation of benzimidazoles and spirooxindoles by an immobilized chlorophyll b on magnetic nanoparticles. Appl Organomet Chem. https://doi.org/10.1002/aoc.5997
Anjaneyulu B, Dharma Rao GB, Nagakalyan S (2021) Synthesis and DFT studies of 1,2-disubstituted benzimidazoles using expeditious and magnetically recoverable CoFe2O4/Cu(OH)2 nanocomposite under solvent-free condition. J Saudi Chem Soc. https://doi.org/10.1016/j.jscs.2021.101394
Kazemi M, Mohammadi M (2020) Magnetically recoverable catalysts: catalysis in synthesis of polyhydroquinolines. Appl Organomet Chem 34:e5400. https://doi.org/10.1002/aoc.5400
Mohammadi M, Ghorbani-Choghamarani A (2022) A novel Hercynite-supported tetradentate Schiff base complex of manganese catalyzed one-pot annulation reactions. Appl Organomet Chem 36:e6905. https://doi.org/10.1002/aoc.6905
Zhao Y (2022) Co-precipitated Ni/Mn shell coated nano Cu-rich core structure: a phase-field study. J Mater Res Technol 21:546–560. https://doi.org/10.1016/j.jmrt.2022.09.032
Borade RM, Kale SB, Tekale SU et al (2021) Cobalt ferrite magnetic nanoparticles as highly efficient catalyst for the mechanochemical synthesis of 2-aryl benzimidazoles. Catal Commun 159:106349. https://doi.org/10.1016/j.catcom.2021.106349
Shah Hosseini M, Ghafuri H, EsmailiZand HR (2018) 4,5-Imidazoledicarboxylic acid immobilized on Fe 3 O 4 magnetic nanoparticles: Preparation, characterization, and application as a recyclable and efficient nanocatalyst in the sonochemical condensation reaction. J Chinese Chem Soc 65:850–855. https://doi.org/10.1002/jccs.201700455
Dezfoolinezhad E, Ghodrati K, Badri R (2016) Fe 3 O 4 @SiO 2 @polyionene/Br 3−core–shell–shell magnetic nanoparticles: a novel catalyst for the synthesis of imidazole derivatives under solvent-free conditions. New J Chem 40:4575–4587. https://doi.org/10.1039/C5NJ02680F
Kazemi M (2020) Magnetically reusable nanocatalysts in biginelli synthesis of dihydropyrimidinones (DHPMs). Synth Commun 50:1409–1445. https://doi.org/10.1080/00397911.2020.1720740
Norouzi M, Noormoradi N, Mohammadi M (2023) Nanomagnetic tetraaza (N4 Donor) macrocyclic schiff base complex of copper(II): synthesis, characterizations, and its catalytic application in click reaction. Nanoscale Adv. https://doi.org/10.1039/D3NA00580A
Mousavi-Mashhadi SA, Shiri A (2021) On-water and efficient ullmann-type O-arylation cross coupling reaction of phenols and aryl tosylates in the presence of Fe 3 O 4 @starch-Au as nanocatalyst. ChemistrySelect 6:3941–3951. https://doi.org/10.1002/slct.202004327
Mazraati A, Setoodehkhah M, Moradian M (2023) Synthesis of copper(II) Schiff base complex immobilized on magnetite-silica nanoparticles and using as a reusable catalyst for the synthesis of 1-amidoalkyl-2-naphthols under ultrasonic conditions. J Clust Sci. https://doi.org/10.1007/s10876-023-02456-1
Li W, Yan J, Xu W, Zhang LY (2023) Magnetic nanoparticles modified with a copper( <scp>i</scp> ) complex as a novel and efficient reusable catalyst for A 3 coupling leading to C–N bond formation. RSC Adv 13:28964–28974. https://doi.org/10.1039/D3RA04871C
Bertolucci E, Galletti AMR, Antonetti C et al (2015) Chemical and magnetic properties characterization of magnetic nanoparticles. 2015 IEEE international instrumentation and measurement technology conference (I2MTC) proceedings. IEEE, pp 1492–1496
RasulMousavi M, Aboonajmi J, TaherMaghsoodlou M et al (2013) La(NO3)3.6H2O catalyzed one-pot highly diastereoselective synthesis of functionalized piperidines. Lett Org Chem 10:171–177. https://doi.org/10.2174/1570178611310030005
Khan MM, Khan S, Iqbal S et al (2016) Synthesis of functionalized dihydro-2-oxypyrroles and tetrahydropyridines using 2,6-pyridinedicarboxylic acid as an efficient and mild organocatalyst. New J Chem 40:7504–7512. https://doi.org/10.1039/C6NJ01170E
Brahmachari G, Das S (2012) Bismuth nitrate-catalyzed multicomponent reaction for efficient and one-pot synthesis of densely functionalized piperidine scaffolds at room temperature. Tetrahedron Lett 53:1479–1484. https://doi.org/10.1016/j.tetlet.2012.01.042
Aboonajmi J, Maghsoodlou MT, Hazeri N et al (2015) Tartaric acid: a natural, green and highly efficient catalyst for the one-pot synthesis of functionalized piperidines. Res Chem Intermed 41:8057–8065
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, Z.Y., Wei, W. & Zhang, L.Y. Construction and Characterization of Magnetic Fe3O4 Nanoparticles Supported Zn Complex: Research on Multicomponent Synthesis of Highly Functionalized Piperidines. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04580-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04580-7