Abstract
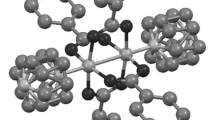
Polyoxometalates can be tuned for specific catalytic property by substituting transition metal ions. We report the synthesis of hybrid materials of Cu2+, Co2+ and Ni2+ substituted phosphotungstates and poly(diallyldimethylammonium) chloride polymer (PDDA) for CO2 fixation. The in situ generated transition metal substituted polyoxometalates (TMS-POMs) are analyzed by FTIR, powder XRD, 31P NMR and SEM techniques. The hybrid TMS-POM materials are found to be good catalysts for converting epoxides to cyclic carbonates. Among these, PDDA-PWCo is the most efficient catalyst for cycloaddition of CO2 under solvent-free conditions at room temperature in shortest reaction time. Only 0.2 mol% of PDDA-PWCo is enough to deliver 100% conversion and selectivity to cyclic carbonates. This catalytic approach is employed for conversion of other cyclic, acyclic, and aromatic epoxides without using column purifications. Overall, the method of obtaining cyclic carbonates under green conditions using TMS-POMs-PDDA hybrid materials appears suitable for industrial applications.
Graphical Abstract













Similar content being viewed by others
References
Truong CC, Mishra DK (2020) Environ Chem Lett 19:911–940. https://doi.org/10.1007/s10311-020-01121-7
Osman AI, Hefny M, Abdel Maksoud MIA, Elgarahy AM, Rooney DW (2020) Environ Chem Lett 19:797–849. https://doi.org/10.1007/s10311-020-01133-3
Peng J, Geng Y, Yang HJ, He W, Wei Z, Yang J, Guo C-Y (2017) Mol Catal 432:37–46. https://doi.org/10.1016/j.mcat.2017.01.019
Chen X, Liu Y, Wu J (2020) Mol Catal 483:110716. https://doi.org/10.1016/j.mcat.2019.110716
Corma A, Garcia H (2013) J Catal 308:168–175. https://doi.org/10.1016/j.jcat.2013.06.008
Zhang YY, Yang GW, Xie R, Yang L, Li B, Wu GP (2020) Angew Chem Int Ed 59:23291–23298. https://doi.org/10.1002/anie.202010651
Lang X-D, He LN (2016) Chem Rec 16:1337–1352. https://doi.org/10.1002/tcr.201500293
Zhai G, Liu Y, Lei L, Wang J, Wang Z, Zheng Z, Wang P, Cheng H, Dai Y, Huang B (2021) ACS Catal 11:1988–1994. https://doi.org/10.1021/acscatal.0c05145
Miao CX, Wang JQ, Wu Y, Du Y, He LN (2008) Chemsuschem 1:236–241. https://doi.org/10.1002/cssc.200700133
Cao JP, Xue YS, Li NF, Gong JJ, Kang RK, Xu Y (2006). J Am Chem Soc. https://doi.org/10.1201/9781420015751
Mizuno N, Kamata K, Yamaguchi K (2006) Surface and Nanomolecular Catalysis. In: Richards Ryan (ed) Liquid-phase oxidations catalysed by polyoxometalates. CRC Press Taylor and Francis, Boca Raton, pp 463–492
Chen F, Li X, Wang B, Xu T, Chen SL, Liu P, Hu C (2012) Chem Eur J 18:9870–9876. https://doi.org/10.1002/chem.201201042
Wang Y, Wu Z, Yu H, Han S, Wei Y (2020) Green Chem 22:3150–3154. https://doi.org/10.1039/d0gc00388c
Yu B, Zou B, Hu CW (2018) J CO2Util 26:314–322. https://doi.org/10.1016/j.jcou.2018.05.021
Wang SS, Yang GY (2015) Chem Rev 115:4893–4962. https://doi.org/10.1021/cr500390v
Miras HN, Yan J, Long DL, Cronin L (2012) Chem Soc Rev 41:7403. https://doi.org/10.1039/c2cs35190k
Pattnaik F, Tripathi S, Patra BR, Nanda S, Kumar V, Dalai AK, Naik S (2021) Environ Chem Lett 19:4119–4136. https://doi.org/10.1007/s10311-021-01284-x
Long DL, Tsunashima R, Cronin L (2010) Angew Chem Int Ed 49:1736–1758. https://doi.org/10.1002/anie.200902483
Nsouli NH, Ismail AH, Helgadottir IS, Dickman MH, Clemente-Juan JM, Kortz U (2009) Inorg Chem 48:5884–5890. https://doi.org/10.1021/ic900180x
Xiao Y, Chen D, Ma N, Hou Z, Hu M, Wang C, Wang W (2013) RSC Adv 3:21544. https://doi.org/10.1039/c3ra43373k
Qi W, Wu L (2009) Polym Int 58:1217–1225. https://doi.org/10.1002/pi.2654
Chen H, Wang Y, Dong S (2007) Inorg Chem 46:10587–10593. https://doi.org/10.1021/ic7009572
Ge W, Wang X, Zhang L, Du L, Zhou Y, Wang J (2016) Catal Sci Technol 6:460–467. https://doi.org/10.1039/c5cy01038a
Zhao YQ, Liu YY, Ma JF (2020) Cryst Growth Des 21:1019–1027. https://doi.org/10.1021/acs.cgd.0c01353
Shakeela K, Sinduri VL, Ranga Rao G (2017) Polyhedron 137:43–51. https://doi.org/10.1016/j.poly.2017.07.023
Shakeela K, Ranga Rao G (2018) ACS Appl Nano Mater 1:4642–4651. https://doi.org/10.1021/acsanm.8b00920
Houston JE, Patterson AR, Jayasundera AC, Schmitt W, Evans RC (2014) Chem Commun 50:5233–5235. https://doi.org/10.1039/c3cc47552b
Zhao W, Yang C, Cheng Z, Zhang Z (2016) Green Chem 18:995–998. https://doi.org/10.1039/c5gc02527c
Shakeela K, Guru S, Ranga Rao G (2020). J Chem Sci. https://doi.org/10.1007/s12039-020-01804-2
Han F, Li H, Zhuang H, Hou Q, Yang Q, Zhang B, Miao C (2021) J CO2 Util 53:101742. https://doi.org/10.1016/j.jcou.2021.101742
Simões MMQ, Conceição CMM, Gamelas JAF, Domingues PMDN, Cavaleiro AMV, Cavaleiro JAS, Ferrer-Correia AJV, Johnstone RAW (1999) J Mol Catal A Chem 144:461–468. https://doi.org/10.1016/s1381-1169(99)00025-4
Zhang Q, An Q, Luan X, Huang H, Li X, Meng Z, Tong W, Chen X, Chu PK, Zhang Y (2015) Nanoscale 7:14002–14009. https://doi.org/10.1039/c5nr03256c
Rajkumar T, Ranga Rao G (2009) J Chem Sci 120:587–594. https://doi.org/10.1007/s12039-008-0089-x
Levine DJ, Stöhr J, Falese LE, Ollesch J, Wille H, Prusiner SB, Long JR (2015) ACS Chem Biol 10:1269–1277. https://doi.org/10.1021/cb5006239
Patel K, Shringarpure P, Patel A (2010) Transit Met Chem 36:171–177. https://doi.org/10.1007/s11243-010-9450-2
Lu X-B, Wang Y (2004) Angew Chem Int Ed 43:3574–3577. https://doi.org/10.1002/anie.200453998
Singh C, Mukhopadhyay S, Das S (2018) Inorg Chem 57:6479–6490. https://doi.org/10.1021/acs.inorgchem.8b00541
Mulkapuri S, Ravi A, Das S (2022) Chem Mater 34:3624–3636. https://doi.org/10.1021/acs.chemmater.1c03917
Zalomaeva OV, Chibiryaev AM, Kovalenko KA, Kholdeeva OA, Balzhinimaev BS, Fedin VP (2013) J Catal 298:179–185. https://doi.org/10.1016/j.jcat.2012.11.029
Das SK, Chatterjee S, Bhunia S, Mondal A, Mitra P, Kumari V, Pradhan A, Bhaumik A (2017) Dalton Trans 46:13783–13792. https://doi.org/10.1039/c7dt02040f
Cheng W, Xue Y, Luo X-M, Xu Y (2018) Chem Commun 54:12808–12811. https://doi.org/10.1039/c8cc07041e
Jia J, Niu Y, Zhang P, Zhang D, Ma P, Zhang C, Niu J, Wang J (2017) Inorg Chem 56:10131–10134. https://doi.org/10.1021/acs.inorgchem.7b01231
Lu J, Ma X, Singh V, Zhang Y, Wang P, Feng J, Ma P, Niu J, Wang J (2018) Inorg Chem 57:14632–14643. https://doi.org/10.1021/acs.inorgchem.8b02321
Szczepankiewicz SH, Ippolito CM, Santora BP, Van de Ven TJ, Ippolito GA, Fronckowiak L, Wiatrowski F, Power T, Kozik M (1998) Inorg Chem 37:4344–4352. https://doi.org/10.1021/ic980162k
Khenkin AM, Efremenko I, Weiner L, Martin JML, Neumann R (2010) Chem Eur J 16:1356–1364. https://doi.org/10.1002/chem.200901673
Acknowledgements
RJ would like to express gratitude to Prof. Ranga Rao for providing research facilites to carry out this work in DSEHC-Solar Fuels Laboratory at IIT Madras. DSEHC is supported by Department of Science and Technology, Government of India through Grant No. DST/TMD/SERI/HUB/1(C). RJ, CAB, RRS and KS acknowledge BSACIST for research faciltites for a part of this work.
Author information
Authors and Affiliations
Contributions
RJ, CAB: material preparation, data collection, early draft and analysis; RRS: study conception; KS: materials design; RRS and KS: study design, monitoring, data analysis, manuscript draft preparation, editing; GRR: Funding, study design, monitoring and final editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant conflicts of interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jan, R., Biji, C.A., Shakeela, K. et al. Green Synthesis of Cyclic Carbonates from Epoxides and CO2 Using Transition Metal Substituted Polyoxometalate-PDDA Hybrid Catalysts. Catal Lett 154, 1631–1641 (2024). https://doi.org/10.1007/s10562-023-04392-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04392-1