Abstract
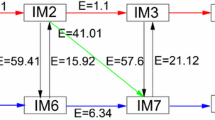
In this work, density functional theory is used to study the mechanism of propane dehydrogenation over non-metallic C3N catalyst. The structure, electrostatic potential and density of state of C3N are introduced, as well as the adsorption of reactants on catalyst is studied. The propane dehydrogenation reaction is divided into the first dehydrogenation and the second dehydrogenation (deep dehydrogenation). We explore the possible dehydrogenation pathways in two-step dehydrogenation. The rate control step of the first dehydrogenation is the removal of methylene hydrogen atom from propane, and its energy barrier is 47.79 kcal/mol, which reflected the catalytic activity of the catalyst. The rate control step of deep dehydrogenation is the process of removing the first hydrogen atom of the product propylene to produce the by-product. The energy barrier is 72.80 kcal/mol, which is much larger than that of the first step of dehydrogenation, reflecting the excellent selectivity of the catalyst.
Graphic Abstract








Similar content being viewed by others
References
Zhang Y, Yang S, Lu J, Mei Y, He D, Luo Y (2019) Effect of a ce promoter on nonoxidative dehydrogenation of propane over the commercial Cr/Al2O3 catalyst. IndEngChem Res 58(43):19818–19824. https://doi.org/10.1021/acs.iecr.9b03870
Zhang Y, Zhou Y, Qiu A, Wang Y, Xu Y, Wu P (2006) Propane dehydrogenation on PtSn/ZSM-5 catalyst: effect of tin as a promoter. CatalCommun 7(11):860–866. https://doi.org/10.1016/j.catcom.2006.03.016
Fu H, Liu ZP, Li ZH, Wang WN, Fan KN (2006) Periodic density functional theory study of propane oxidative dehydrogenation over V2O5(001) surface. J Am ChemSoc 128(34):11114–11123. https://doi.org/10.1021/ja0611745
Zhang Y, Zhou Y, Shi J, Zhou S, Sheng X, Zhang Z, Xiang S (2014) Comparative study of bimetallic Pt Sn catalysts supported on different supports for propane dehydrogenation. J MolCatal A Chem 381:138–147. https://doi.org/10.1016/j.molcata.2013.10.007
Gao XQ, Lu WD, Hu SZ, Li WC, Lu AH (2019) Rod-shaped porous alumina-supported Cr2O3 catalyst with low acidity for propane dehydrogenation. Chin J Catal 40(2):184–191. https://doi.org/10.1016/s1872-2067(18)63202-4
Liu J, Yue Y, Liu H, Da Z, Liu C, Ma A, Rong J, Su D, Bao X, Zheng H (2017) Origin of the robust catalytic performance of nanodiamond–graphene-supported pt nanoparticles used in the propane dehydrogenation reaction. ACS Catal 7(5):3349–3355. https://doi.org/10.1021/acscatal.6b03452
Bauer T, Maisel S, Blaumeiser D, Vecchietti J, Taccardi N, Wasserscheid P, Bonivardi A, Görling A, Libuda J (2019) Operando DRIFTS and DFT study of propane dehydrogenation over solid- and liquid-supported gaxpty catalysts. ACS Catal 9(4):2842–2853. https://doi.org/10.1021/acscatal.8b04578
Sattler JJHB, Ruiz-Martinez J, Santillan-Jimenez E, Weckhuysen BM (2014) Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem Rev 114(20):10613–10653. https://doi.org/10.1021/cr5002436
Yang ML, Zhu J, Zhu YA, Sui ZJ, Yu YD, Zhou XG, Chen D (2014) Tuning selectivity and stability in propane dehydrogenation by shaping Pt particles: a combined experimental and DFT study. J MolCatalAChem 395:329–336. https://doi.org/10.1016/j.molcata.2014.08.008
Yarusov IB, Zatolokina EV, Shitova NV, Belyi AS, Ostrovskii NM (1992) Propane dehydrogenation over Pt-Sn catalysts. Catal Today 13(4):655–658. https://doi.org/10.1016/0920-5861(92)80105-V
Bariås OA, Holmen A, Blekkan EA (1996) Propane dehydrogenation over supported Pt and Pt-Sn catalysts: catalyst preparation,characterization and activity measurements. J Catal 158(1):1–12. https://doi.org/10.1006/jcat.1996.0001
Takehira K, Ohishi Y, Shishido T, Kawabata T, Takaki K, Zhang Q, Wang Y (2004) behavior of active sites on Cr-MCM-41 catalysts during the dehydrogenation of propane with CO2. J Catal 224(2):404–416. https://doi.org/10.1016/j.jcat.2004.03.014
Yu C, Ge Q, Xu H, Li W (2006) Effects of Ce addition on the Pt-Sn/γ-Al2O3 catalyst for propane dehydrogenation to propylene. ApplCatal A Gen 315:58–67. https://doi.org/10.1016/j.apcata.2006.08.038
Shishido T, Shimamura K, Teramura K, Tanaka T (2012) Role of CO2 in dehydrogenation of propane over Cr-based catalysts. Catal Today 185(1):151–156. https://doi.org/10.1016/j.cattod.2011.10.028
Zhu Y, An Z, Song H, Xiang X, Yan W, He J (2017) Lattice-confined Sn (IV/II) stabilizing raft-like pt clusters: high selectivity and durability in propane dehydrogenation. ACS Catal 7(10):6973–6978. https://doi.org/10.1021/acscatal.7b02264
Hu ZP, Yang D, Wang Z, Yuan ZY (2019) State-of-the-art catalysts for direct dehydrogenation of propane to propylene. Chin J Catal 40(9):1233–1254. https://doi.org/10.1016/S1872-2067(19)63360-7
Sun P, Siddiqi G, Vining WC, Chi M, Bell AT (2011) Novel Pt/Mg(In)(Al)O catalysts for ethane and propane dehydrogenation. J Catal 282(1):165–174. https://doi.org/10.1016/j.jcat.2011.06.008
Santhosh Kumar M, Hammer N, Rønning M, Holmen A, Chen D, Walmsley JC, Øye G (2009) The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: new insights by a comprehensive spectroscopic study. J Catal 261(1):116–128. https://doi.org/10.1016/j.jcat.2008.11.014
Otroshchenko TP, Rodemerck U, Linke D, Kondratenko EV (2017) Synergy effect between Zr and Cr active sites in binary CrZrOx or supported CrOx/LaZrOx: consequences for catalyst activity, selectivity and durability in non-oxidative propane dehydrogenation. J Catal 356:197–205. https://doi.org/10.1016/j.jcat.2017.10.012
Mitchell PCH, Wass SA (2002) Propane dehydrogenation over molybdenum hydrotalcite catalysts. ApplCatal A Gen 225(1):153–165. https://doi.org/10.1016/S0926-860X(01)00862-6
Hu B, Schweitzer NM, Zhang G, Kraft SJ, Childers DJ, Lanci MP, Miller JT, Hock AS (2015) Isolated feii on silica as a selective propane dehydrogenation catalyst. ACS Catal 5(6):3494–3503. https://doi.org/10.1021/acscatal.5b00248
Camacho-Bunquin J, Aich P, Ferrandon M, Bean Getsoian A, Das U, Dogan F, Curtiss LA, Miller JT, Marshall CL, Hock AS, Stair PC (2017) Single-site zinc on silica catalysts for propylene hydrogenation and propane dehydrogenation: synthesis and reactivity evaluation using an integrated atomic layer deposition-catalysis instrument. J Catal 345:170–182. https://doi.org/10.1016/j.jcat.2016.10.017
Cybulskis VJ, Pradhan SU, Lovón-Quintana JJ, Hock AS, Hu B, Zhang G, Delgass WN, Ribeiro FH, Miller JTJCL (2017) The nature of the isolated gallium active center for propane dehydrogenation on Ga/SiO2. CatalLett 147(5):1252–1262. https://doi.org/10.1007/s10562-017-2028-2
Schweitzer NM, Hu B, Das U, Kim H, Greeley J, Curtiss LA, Stair PC, Miller JT, Hock AS (2014) Propylene hydrogenation and propane dehydrogenation by a single-site Zn2+ on silica catalyst. ACS Catal 4(4):1091–1098. https://doi.org/10.1021/cs401116p
Raman N, Maisel S, Grabau M, Taccardi N, Debuschewitz J, Wolf M, Wittkämper H, Bauer T, Wu M, Haumann M, Papp C, Görling A, Spiecker E, Libuda J, Steinrück H-P, Wasserscheid P (2019) Highly effective propane dehydrogenation using Ga–Rh supported catalytically active liquid metal solutions. ACS Catal 9(10):9499–9507. https://doi.org/10.1021/acscatal.9b02459
Kim Wg, So J, Choi SW, Liu Y, Dixit RS, Sievers C, Sholl DS, Nair S, Jones CW (2017) Hierarchical Ga-MFI catalysts for propane dehydrogenation. Chem Mater 29(17):7213–7222. https://doi.org/10.1021/acs.chemmater.7b01566
Yang ML, Zhu YA, Fan C, Sui ZJ, Chen D, Zhou XG (2011) DFT study of propane dehydrogenation on Pt catalyst: effects of step sites. PhysChemChemPhys 13(8):3257. https://doi.org/10.1039/c0cp00341g
Yang ML, Zhu YA, Zhou XG, Sui ZJ, Chen D (2012) First-principles calculations of propane dehydrogenation over PtSn catalysts. ACS Catal 2(6):1247–1258. https://doi.org/10.1021/cs300031d
Cao X, Ji Y, Luo Y (2015) Dehydrogenation of propane to propylene by a Pd/Cu single-atom catalyst: insight from first-principles calculations. J PhysChem C 119(2):1016–1023. https://doi.org/10.1021/jp508625b
Tuo Y, Yang L, Cheng H, Yang M, Zhu YA, Li P (2018) Density functional theory study of decalin dehydrogenation for hydrogen release on Pt(111)and Pt(211). Int J Hydrog Energy 43(42):19575–19588. https://doi.org/10.1016/j.ijhydene.2018.09.002
Sun X, Liu M, Huang Y, Li B, Zhao Z (2019) Electronic interaction between single Pt atom and vacancies on boron nitride nanosheets and its influence on the catalytic performance in the direct dehydrogenation of propane. Chin J Catal 40(6):819–825. https://doi.org/10.1016/S1872-2067(18)63196-1
Song Y, Liu G, Yuan ZY (2016) N-, P- and B-doped mesoporous carbons for direct dehydrogenation of propane. RSC Adv 6(97):94636–94642. https://doi.org/10.1039/c6ra20726j
Frank B, Zhang J, Blume R, Schlogl R, Su DS (2009) Heteroatoms increase the selectivity in oxidative dehydrogenation reactions on nanocarbons. AngewChemInt Ed Engl 48(37):6913–6917. https://doi.org/10.1002/anie.200901826
Khavryuchenko OV, Frank B, Trunschke A, Hermann K, Schlögl R (2013) Quantum-chemical investigation of hydrocarbon oxidative dehydrogenation over spin-active carbon catalyst clusters. J PhysChem C 117(12):6225–6234. https://doi.org/10.1021/jp312548g
Grant JT, Carrero CA, Goeltl F, Venegas J, Mueller P, Burt SP, Specht SE, McDermott WP, Chieregato A, Hermans I (2016) Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts. Science 354(6319):1570–1573. https://doi.org/10.1126/science.aaf7885
Sun X, Li B, Su D (2016) The unexpected reactivity of the carbon sites on the nanostructured carbon catalysts towards the C−H Bond activation from the analysis of the aromaticity. Chem -Asian J 11(11):1668–1671. https://doi.org/10.1002/asia.201600222
Li X, Zhu L, Xue Q, Chang X, Ling C, Xing WJA (2017) Superior selective CO2 adsorption of C3N pores: GCMC and DFT simulations. ACS Appl Mater Interfaces 9(36):31161–31169. https://doi.org/10.1021/acsami.7b09648
Makaremi M, Mortazavi B, Singh CVJTJ (2017) Adsorption of metallic, metalloidic, and nonmetallic adatoms on two-dimensional C3N. J PhysChem C 121(34):18575–18583. https://doi.org/10.1021/acs.jpcc.7b04511
Bafekry A, FarjamiShayesteh S, Peeters FMJTJ (2019) C3N monolayer: exploring the emerging of novel electronic and magnetic properties with adatom adsorption, functionalizations, electric field, charging, and strain. J PhysChem C 123(19):12485–12499. https://doi.org/10.1021/acs.jpcc.9b02047
Mortazavi B (2017) Ultra high stiffness and thermal conductivity of graphene like C3N. Carbon 118:25–34. https://doi.org/10.1016/j.carbon.2017.03.029
Frisch M, Trucks G, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GJI (2009) Gaussian 09, Revision d 01. Gaussian, Wallingford CT, p 201
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789. https://doi.org/10.1103/PhysRevB.37.785
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J ChemPhys 132:15. https://doi.org/10.1063/1.3382344
Van Duijneveldt FB, van Duijneveldt-vandeRijdt JG, van Lenthe JHJCR (1994) State of the art in counterpoise theory. Chem Rev 94(7):1873–1885
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J ChemPhys 90(4):2154–2161. https://doi.org/10.1063/1.456010
Gonzalez C, Schlegel HBJJ (1990) Reaction path following in mass-weighted internal coordinates. J PhysChem 94(14):5523–5527
Shi LB, Zhang YY, Xiu XM, Dong HK (2018) Carbon 134:103–111. https://doi.org/10.1016/j.carbon.2018.03.076
Yang S, Li W, Ye C, Wang G, Tian H, Zhu C, He P, Ding G, Xie X, Liu Y, Lifshitz Y, Lee ST, Kang Z, Jiang M (2017) C3N—A 2D crystalline, hole-free, tunable-narrow-bandgap semiconductor with ferromagnetic properties. Adv Mater 29(16):1605625. https://doi.org/10.1002/adma.201605625
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J ComputChem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Caspary KJ, Gehrke H, Heinritz-Adrian M, Schwefer M (2008) Dehydrogenation of alkanes. Wiley, Hoboken, pp 3206–3229
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, X., Kang, L. & Ren, W. C3N Non-metallic Catalyst for Propane Dehydrogenation: A Density Functional Theory Study. Catal Lett 151, 3154–3164 (2021). https://doi.org/10.1007/s10562-021-03564-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03564-1