Abstract
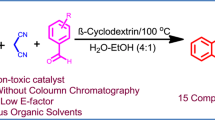
A pragmatic and swift method for the synthesis of Benzo[a]pyrano[2,3-c]phenazine derivatives via one pot, multicomponent strategy by employing β-cyclodextrin in EtOH:H2O (1:1) solvent at 70 °C has been documented here. Utilization of supramolecular catalyst β-cyclodextrin which is highly efficient, green, biodegradable and reusable catalyst augments the synthesis amazingly, is the key feature of the current pathway. The catalyst could be recovered for four successive cycles without significant loss in catalytic activity.
Graphic Abstract








Similar content being viewed by others
References
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, New York
Horvath IT (2002) Acc Chem Res 35:685
Madhumitha G, Roopan SM (2013) J Nanomater 2013:1
Maity A, Chakraborty D, Hazra A, Bharitkar YP, Kundu S, Maulik PR, Mondal NB (2014) Tetrahedron Lett 55:3059–3063
Srivastava M, Singh J, Singh SB, Tiwari K, Pathak VK, Singh J (2012) Green Chem 14:901–905
Chaudhari MA, Gujar JB, Kawade DS, Shinde PV, Shingare MS (2015) Res Chem Intermed 41:10027–10035
Sun T, Wang Q, Bi Y, Chen X, Liu L, Ruan C, Zhao Z, Jiang C (2017) J Mater Chem B5:2644–2654
Ao M, Gan C, Shao W, Xing Z, Yong C (2016) Eur J Pharm Sci 91:183–189
Mishra NP, Mohapatra S, Pravati P, Nayak S (2018) Curr Org Chem 22:1956–1982
Abbasi M (2017) J Chin Chem Soc 64:896–917
Rekharsky MV, Inoue Y (1998) Chem Rev 98:1875–1917
Connors KA (1997) Chem Rev 97:1325–1358
Valle EMD (2004) Biochem 39:1033–1046
Bender ML, Komiyama M (2012) Cyclodextin chemistry. Springer Science & Business Media, Berlin
Seidi F, Ahmad AS, Mojtaba A, Meisam S, Daniel C (2019) Polym Chem 10:3674–3711
Kumar AR, Ashok K, Brahmaiah B, Sreekanth N, Baburao C (2013) IJPRBS 2(2):291–304
Rutenberg R, Leitus G, Fallik E, Poverenov E (2016) Chem Commun 52:2565–2568
Szejtli J (1998) Chem Rev 98:1743–1754
Ramón DJ, Yus M (2005) Angew Chem Int Ed 44:1602–1634
Voigt B, Linke M, Mahrwald R (2015) Org Lett 17:2606–2609
Toure BB, Hall DG (2009) Chem Rev 109:4439–4486
Alvim HGO, Silva JE, Neto BAD (2014) RSC Adv 4:54282–54299
Barreiros ALBS, David JM, David JP (2006) Quim Nova 29:113–123
Muller M, Sorrell T (1995) Prostaglandins 50:301–311
Andrade-Nieto V, Goulart M, da Silva JF, da Silva MJ, Pinto M, Pinto A, Zalis M, Carvalho L, Krettli A (2004) Bioorg Med Chem Lett 14:1145–1149
Ligon J, Dwight S, Hammer P, Torkewitz N, Hofmann D, Kempf H, Pee K (2000) Pest Manage Sci 56:688–695
Vickr N, Burgess L, Chuckowree IS, Dodd R, Folkes AJ, Hardick DJ, Hancox TC, Miller WH, Milton J, Sohal S, Wang S, Wren SP, Charlton PA, Dangerefield W, Liddle C, Mistry P, Stewart AJ, Denny WA (2002) J Med Chem 45:721–739
Neves-Pinto C, Malta V, Pinto M, Santos R, Castro S, Pinto A (2002) J Med Chem 45:740–743
Kondratyuk TP, Park EJ, Yu R, Van BRB, Asolkar RN, Murphy BT, Fenical W, Pezzuto JM (2012) Mar Drugs 10:451–464
Esmaeilpour M, Sardarian AR, Firouzabadi H (2018) ChemistrySelect 3:9236–9248
Chaniyara R, Thakrar S, Kakadiya R, Marvania B, Detroja D, Vekariya N, Upadhyay K, Manvar A, Shah A (2014) J Hetercyclic Chem 51:466–474
Magedov IV, Manpadi M, Ogasawara MA, Dhawan AS, Rogelj S, Van Slambrouck S, Steelant WFA, Evdokimov NM, Uglinskii PY, Elias EM, Knee EJ, Tongwa P, Antipin MY, Kornienko A (2008) J Med Chem 51:2561–2570
Narender T, Gupta S (2004) Bioorg Med Chem Lett 14:3913–3916
Evidente A, Cabras A, Maddau L, Serra S, Andolfi A, Motta AJ (2003) Agr Food Chem 51:6957–6960
Aytemir MD, Calis U, Ozalp M (2004) Arch Pharm Pharm Med Chem 337:281–288
Esmaeilpour M, Javidi J, Dehghani F, Dodeji FN (2015) RSC Adv 5:26625–26633
Wang SL, Wu FY, Cheng C, Zhang G, Liu YP, Jiang B, Shi F, Tu SJ (2011) ACS Comb Sci 13:135–139
Shaterian HR, Moradi F, Mohammadnia M (2012) C R Chim 15:1055–1059
Shaterian HR, Mohammadnia M (2013) J Mol Liq 177:162–166
Bharti R, Parvin T (2016) Mol Divers 20:867–876
Hasaninejad A, Firoozi S (2013) Mol Divers 17:499–513
Choghamarani AG, Sahraei R, Taherinia Z (2019) Res Chem Intermed 45(5):3199–3214
Yazdani-Elah-Abadi A, Maghsoodlou MT, Mohebat R, Heydari R (2017) Chin Chem Lett 28(2):446–452
Gao J, Chen M, Tong X, Zhu H, Yan H, Liu D, Li W, Qi S, Xiao D, Wang Y, Lu Y, Jiang F (2015) Comb Chem High Throughput Screen 18(10):960–974
Zarabi MF, Naeimi H (2019) Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2019.1672202
Choghamarani AG, Mohammadi M, Shiri L, Taherinia Z (2019) Res Chem Intermed 45:5705–5723
Naeimi H, Zarabi MF (2019) RSC Adv 9:7400–7410
Rai P, Srivastava M, Yadav S, Singh J, Singh J (2015) Catal Lett 145:2020–2028
Mishra A, Srivastava M, Rai P, Yadav S, Tripathi BP, Singh J, Singh J (2016) RSC Adv 6:49164–49172
Mishra A, Rai P, Srivastava M, Tripathi BP, Yadav S, Singh J, Singh J (2017) Catal Lett 147:2600–2611
Tripathi BP, Mishra A, Rai P, Pandey YK, Srivastava M, Yadav S, Singh J, Singh J (2017) New J Chem 41:11148–11154
Mishra A, Rai P, Singh J, Singh J (2018) ChemistrySelect 3:8408–8414
Mishra A, Rai P, Pandey YK, Singh J, Singh J (2017) ChemistrySelect 2:10979–10983
Tayade YA, Dalal DS (2017) Catal Lett 147:1411–1421
Ghorad A, Mahalle S, Khillare LD, Sangshetti JN, Bhosle MR (2017) Catal Lett 147:640–648
Acknowledgements
The authors are thankful to SAIF, Punjab University, Chandigarh, India for providing spectral data. Authors A. Mishra, Y. K. Pandey and F. Tufail are thankful to UGC and CSIR, New Delhi, for the award of Senior Research Fellowship (SRF). Prof. J. Singh thankful to UGC for UGC BSR fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mishra, A., Pandey, Y.K., Tufail, F. et al. A Convenient and Green Synthetic Approach for Benzo[a]pyrano[2,3-c]phenazines via Supramolecular Catalysis. Catal Lett 150, 1659–1668 (2020). https://doi.org/10.1007/s10562-019-03057-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-03057-2