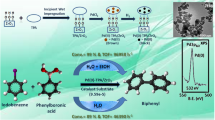
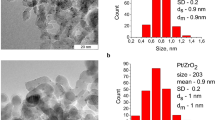
Abstract
Stabilized Pd(0) nanoparticles by supported 12-tungstophosphoric acid (Pd(0)-TPA/ZrO2) was explored as a sustainable recyclable catalyst for selective C=C hydrogenation of cyclohexene and crotonaldehyde. The catalyst shows an outstanding performance [catalyst to substrate ratio (1:1.31 × 104)] towards high conversion as well as 100% selectivity of the desired product with high turnover number (> 10,000) and turnover frequency (> 2600 h−1) for both the systems. The use of neat water as a solvent and mild reaction conditions makes the present system environmentally benign and green. Moreover, the catalyst could be recovered and reused up to five cycles without any significant loss in their conversion as well as selectivity. The viability of the catalyst was evaluated towards different aromatic as well as aliphatic arenes and found to be excellent in all the cases. The obtained selectivity, especially butyraldehyde, was correlated with the nature of the catalyst as well as solvent and based on the study, a plausible mechanism for both the reactions was also proposed.
Graphical Abstract












Similar content being viewed by others
References
Panpranot J, Phandinthong K, Praserthdam P, Hasegawa M, S-i Fujita, Arai M (2006) A comparative study of liquid-phase hydrogenation on Pd/SiO2 in organic solvents and under pressurized carbon dioxide: activity change and metal leaching/sintering. J Mol Catal A 253:20–24
Campo BC, Volpe MA, Gigola CE (2009) Liquid-phase hydrogenation of crotonaldehyde over platinum- and palladium-based catalysts. Ind Eng Chem Res 48:10234–10239
Tao R, Miao S, Liu Z, Xie Y, Han B, An G, Ding K (2009) Pd nanoparticles immobilized on sepiolite by ionic liquids: efficient catalysts for hydrogenation of alkenes and Heck reactions. Green Chem 11:96–101
Zhang T, Li B, Zhang X, Qiu J, Han W, Yeung KL (2014) Pd nanoparticles immobilized in a microporous/mesoporous composite ZIF-8/MSS: a multifunctional catalyst for the hydrogenation of alkenes. Microporous Mesoporous Mater 197:324–330
Puskas R, Sápi A, Kukovecz Á, Kónya Z (2016) Understanding the role of post-CCVD synthetic impurities, functional groups and functionalization-based oxidation debris on the behaviour of carbon nanotubes as a catalyst support in cyclohexene hydrogenation over Pd nanoparticles. RSC Adv 6:88538–88545
Zhang C, Leng Y, Jiang P, Li J, Du S (2017) Immobilizing palladium nanoparticles on nitrogen-doped carbon for promotion of formic acid dehydrogenation and alkene hydrogenation. ChemistrySelect 2:5469–5474
Zhao F, Ikushima Y, Chatterjee M, Shirai M, Arai M (2003) An effective and recyclable catalyst for hydrogenation of α, β-unsaturated aldehydes into saturated aldehydes in supercritical carbon dioxide. Green Chem 5:76–79
Iwasa N, Takizawa M, Arai M (2005) Palladium-based alloy and monometallic catalysts for gas phase hydrogenation of crotonaldehyde: effects of alloying and alloy crystallite size. Appl Catal A 283:255–263
Harraz FA, El-Hout SE, Killa HM, Ibrahim IA (2013) Catalytic hydrogenation of crotonaldehyde and oxidation of benzene over active and recyclable palladium nanoparticles stabilized by polyethylene glycol. J Mol Catal A 370:182–188
Özkar S, Finke RG (2016) Palladium(0) nanoparticle formation, stabilization, and mechanistic studies: Pd(acac)2 as a preferred precursor, [Bu4 N]2HPO4 stabilizer, plus the stoichiometry, kinetics, and minimal, four-step mechanism of the palladium nanoparticle formation and subsequent agglomeration reactions. Langmuir 32:3699–3716
Strimbu L, Liu J, Kaifer AE (2003) Cyclodextrin-capped palladium nanoparticles as catalysts for the Suzuki reaction. Langmuir 19:483–485
Narayanan R, El-Sayed MA (2003) Effect of catalysis on the stability of metallic nanoparticles: suzuki reaction catalyzed by PVP-Palladium nanoparticles. J Am Chem Soc 125:8340–8347
Hamill NA, Hardacre C, McMath SEJ (2002) In situ XAFS investigation of palladium species present during the heck reaction in room temperature ionic liquids. Green Chem 4:139–142
Rocaboy C, Gladysz JA (2002) Highly active thermomorphic fluorous palladacycle catalyst precursors for the heck reaction; evidence for a palladium nanoparticle pathway. Org Lett 4:1993–1996
Li Y, Boone E, El-Sayed MA (2002) Size effects of PVP−Pd nanoparticles on the catalytic Suzuki reactions in aqueous solution. Langmuir 18:4921–4925
Deshmukh RR, Rajagopal R, Srinivasan KV (2001) Ultrasound promoted C-C bond formation: heck reaction at ambient conditions in room temperature ionic liquids. Chem Commun 17:1544–1545
Li Y, El-Sayed MA (2001) The effect of stabilizers on the catalytic activity and stability of Pd colloidal nanoparticles in the suzuki reactions in aqueous solution. Org Lett 105:8938–8943
Moreno-Mañas M, Pleixats R, Villarroya S (2001) Fluorous phase soluble palladium nanoparticles as recoverable catalysts for suzuki cross-coupling and heck reactions. Organometallics 20:4524–4528
Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK (2001) Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc Chem Res 34:181–190
Li Y, Hong XM, Collard DM, El-Sayed MA (2000) Suzuki cross-coupling reactions catalyzed by palladium nanoparticles in aqueous solution. Org Lett 2:2385–2388
Pathak S, Greci MT, Kwong RC, Mercado K, Prakash GKS, Olah GA, Thompson ME (2000) Synthesis and applications of palladium-coated Poly(vinylpyridine) nanospheres. Chem Mater 12:1985–1989
Jana NR, Wang ZL, Pal T (2000) Redox catalytic properties of palladium nanoparticles: surfactant and electron donor−acceptor effects. Langmuir 16:2457–2463
Teranishi T, Miyake M (1998) Size control of palladium nanoparticles and their crystal structures. Chem Mater 10:594–600
Reetz MT, Breinbauer R, Wanninger K (1996) Suzuki and Heck reactions catalyzed by preformed palladium clusters and palladiumnickel bimetallic clusters. Tetrahedron Lett 37:4499–4502
La Sorella G, Sperni L, Canton P, Coletti L, Fabris F, Strukul G, Scarso A (2018) Selective hydrogenations and dechlorinations in water mediated by anionic surfactant-stabilized pd nanoparticles. J Org Chem 83:7438–7446
Kogan V, Aizenshtat Z, Popovitz-Biro R, Neumann R (2002) Carbon−carbon and carbon−nitrogen coupling reactions catalyzed by palladium nanoparticles derived from a palladium substituted Keggin-type polyoxometalate. Organic Lett. 4:3529–3532
Zhang J, Keita B, Nadjo L, Mbomekalle IM, Liu T (2008) Self-assembly of polyoxometalate macroanion-capped Pd0 nanoparticles in aqueous solution. Langmuir 24:5277–5283
D’Souza L, Noeske M, Richards RM, Kortz U (2013) Palladium (0) metal clusters: novel krebs type polyoxoanions stabilized, extremely active hydrogenation catalyst. Appl Catal A 453:262–271
Villanneau R, Roucoux A, Beaunier P, Brouri D, Proust A (2014) Simple procedure for vacant POM-stabilized palladium (0) nanoparticles in water: structural and dispersive effects of lacunary polyoxometalates. RSC Adv 4:26491–26498
Kogan V, Aizenshtat Z, Neumann R (2002) Preferential catalytic hydrogenation of aromatic compounds versus ketones with a palladium substituted polyoxometalate as pre-catalyst. New J Chem 26:272–274
Rana S, Parida KM (2012) A simple and efficient protocol using palladium based lacunary phosphotungstate supported mesoporous silica towards hydrogenation of p-nitrophenol to p-aminophenol at room temperature. Catal Sci Technol 2:979–986
Patel A, Patel A (2018) Stabilized palladium nanoparticles: synthesis, multi-spectroscopic characterization and application for Suzuki–Miyaura reaction. Catal Lett 148:3534–3547
Patel S, Purohit N, Patel A (2003) Synthesis, characterization and catalytic activity of new solid acid catalysts, H3PW12O40 supported on to hydrous zirconia. J Mol Catal A 192:195–202
Bhatt N, Shah C, Patel A (2007) 12-tungstophosphoric and 12-tungstosilicicacid supported onto hydrous zirconia for liquid phase tert-butylation of m-cresol. Catal Lett 117:146–152
Pathan S, Patel A (2012) Heck coupling catalyzed by Pd exchanged supported 12-tunstophosphoric acid—an efficient ligand free, low Pd-loading heterogeneous catalyst. RSC Adv 2:116–120
Militello MC, Simko SJ (1994) Elemental palladium by XPS. Surf Sci Spectra 3:387–394
Abbas Khakiani B, Pourshamsian K, Veisi H (2015) A highly stable and efficient magnetically recoverable and reusable Pd nanocatalyst in aqueous media heterogeneously catalysed Suzuki C-C cross-coupling reactions. Appl Organomet Chem 29:259–265
Patel A, Patel A, Narkhede N (2019) Hydrogenation of cyclohexene in aqueous solvent mixture over a sustainable recyclable catalyst comprising palladium and monolacunary silicotungstate anchored to MCM-41. Eur J Inorg Chem 2019:423–429
Liu J-H, Yang L-M, Ganz E (2018) Efficient and selective electroreduction of CO2 by single-atom catalyst two-dimensional TM–Pc monolayers. ACS Sustain Chem Eng 6:15494–15502
Xu L, Yang L-M, Ganz E (2018) Mn–graphene single-atom catalyst evaluated for CO oxidation by computational screening. Theor Chem Acc 137:98
Ruiz-Martinez J, Fukui Y, Komatsu T, Sepulveda-Escribano A (2008) Ru–Ti intermetallic catalysts for the selective hydrogenation of crotonaldehyde. J Catal 260:150–156
Gallezot P, Richard D (1998) Selective hydrogenation of α, β-unsaturated aldehydes. Catal Rev 40:81–126
Vannice MA, Sen B (1989) Metal-support effects on the intramolecular selectivity of crotonaldehyde hydrogenation over platinum. J Catal 115:65–78
Abid M, Ehret G, Touroude R (2001) Pt/CeO2 catalysts: correlation between nanostructural properties and catalytic behaviour in selective hydrogenation of crotonaldehyde. Appl Catal A 217:219–229
Hubaut R, Daage M, Bonnelle JP (1986) Selective hydrogenation on copper chromite catalysts IV. Hydrogenation selectivity for α, β-unsaturated aldehydes and ketones. Appl Catal 22:231–241
Augustine RL (1976) Organic functional group hydrogenation. Catal Rev 13:285–316
Touroude R (1980) Catalytic behavior of group VIII transition metals in the deuterium-acrolein reaction. J Catal 65:110–120
Shirai M, Tanaka T, Arai M (2001) Selective hydrogenation of α, β-unsaturated aldehyde to unsaturated alcohol with supported platinum catalysts at high pressures of hydrogen. J Mol Catal A 168:99–103
Acknowledgements
We are thankful to Department of Atomic Energy (DAE) and Board of Research in Nuclear Science (BRNS), Project No. 37(2)/14/34/2014-BRNS, Mumbai, for the financial support. One of the authors Mr. Anish Patel is thankful to the same for the grant of JRF. We are also thankful to Department of Chemistry, The Maharaja Sayajirao University of Baroda for BET surface area analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patel, A., Patel, A. Selective C=C Hydrogenation of Unsaturated Hydrocarbons in Neat Water Over Stabilized Palladium Nanoparticles Via Supported 12-Tungstophosphoric Acid. Catal Lett 149, 1476–1485 (2019). https://doi.org/10.1007/s10562-019-02763-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02763-1