Abstract
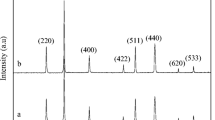
The Fischer–Tropsch synthesis is a catalytic conversion of syngas into hydrocarbon mixtures. A solvothermal method was used to prepare Iron–cobalt–cerium nanocatalyst for conversion of syngas into light olefins. Duration of aging time in preparing method is a key factor affecting in a chemical process. The results showed that the optimal aging time and operating temperature in order to increase light olefin selectivity is at 11 h and 330 °C. The effects of experimental variables including aging time (5, 8 and 11 h) and operating temperature (270–380 °C) were investigated. The nanocatalysts were characterized by temperature programed-reduction (TPR), X-ray diffraction (XRD), energy dispersive X-ray, Fourier infrared spectroscopy, and vibrating sample magnetometer. TPR profiles of the nanocatalysts synthesized at different time of aging indicated that aging time shifted the sample’s reducibility via affecting its particle size. Average crystalline size determined from XRD patterns ranged over 4.5–6.6 nm. It is found that particle size increases with increasing aging time. The results show that time of aging has effect on magnetic properties of nanocatalysts changing from soft ferromagnetic to a hard ferromagnetic one as aging time increases. In addition, the sample aged for 5h exhibits a maximum saturation magnetization of 7.768 emu/g, the maximum coercivity value of 1744.46 Oe is obtained for the sample aged for 8 h.
Graphical Abstract













Similar content being viewed by others
References
Pendyala VRR, Shafer WD, Jacobs G, Davis BH (2014) Fischer–Tropsch synthesis: effect of reaction temperature for aqueous-phase synthesis over a platinum promoted co/alumina catalyst. Catal Lett 144:1088–1095
Pendyala VRR, Shafer WD, Jacobs G, Graham UM, Khalid S, Davis BH (2015) Fischer–Tropsch synthesis: effect of reducing agent for aqueous-phase synthesis over Ru nanoparticle and supported Ru catalysts. Catal Lett 145:893–904
Luo M, Shafer WD, Davis BH (2014) Fischer–Tropsch synthesis: branched paraffin distribution for potassium promoted iron catalysts. Catal Lett 144:1031–1041
Li J, Cheng X, Zhang C, Dong W, Yang Y, Li Y (2016) Comparative study of iron-based Fischer–Tropsch synthesis catalysts promoted with strontium or potassium. Catal Lett 146:2574–2584
Gnanamani MK, Pendyala VRR, Jacobs G, Sparks DE, Shafer WD, Davis BH (2014) Fischer–Tropsch synthesis: effect of halids and potassium addition on activity and selectivity of cobalt. Catal Lett 144:1127–1133
Taherzadeh Lari T, Mirzaei AA, Atashi H (2016) Influence of fabrication temperature and time on light olefin selectivity on iron–cobalt–cerium mixed oxide nanocatalyst for CO hydrogenation. Ind Eng Chem Res. doi:10.1021/acs.iecr.6b03171
Mirzaei A, Vahid S, Feyzi M (2009) Fischer–Tropsch synthesis over iron manganese catalysts: effect of preparation and operating conditions on catalyst performance. Adv Phys Chem. doi:10.1155/2009/151489.
Mirzaei A, Galavy M, Beigbabaei A, Eslamimanesh V (2007) Preparation and operating conditions for cobalt cerium oxide catalysts used in the conversion of synthesis gas into light olefins. J Iran Chem Soc 4:347–363
Mirzaei A, Shahriari S, Arsalanfar M (2011) Effect of preparation conditions on the catalytic performance of Co/Ni catalysts for CO hydrogenation. J Nat Gas Sci Eng 3:537–546
Arsalanfar M, Mirzaei AA, Bozorgzadeh HR (2013) Effect of preparation method on catalytic performance, structure and surface reaction rates of MgO supported Fe–Co–Mn catalyst for CO hydrogenation. J Ind Eng Chem 19:478–487
Torshizi HO, Vahid S, Mirzaei AA (2014) Effect of calcination on the structure and catalytic performance of MgO supported Fe–Co–Ni catalyst for CO hydrogenation. J Nat Gas Sci Eng 17:110–118
Guerrero-Ruiz A, Sepulveda-Escribano A, Rodriguez-Ramos I (1994) Mangesium, vanadium and cerium oxides. Appl Catal A 120:71
Facchini M (2010) A study of cobalt catalysts for Fischer–Tropsch synthesis. Phd theses, University of Glasgow, Glasgow
Davis H (2007) Fischer–Tropsch synthesis: comparison of performance of iron and cobalt catalyst. Ind Eng Chem Res 46:8938–8945
Davies KJ, Wells S, Charles SW (1993) The effect of temperature and oleate adsorption on the growth of maghemite particles. J Magn Magn Mater 122:24–28
Khan A, Smirniotis PG (2008) Relationship between temperature-programmed reduction profile and activity of modified ferrite-based catalysts for WGS reaction. J Mol Catal A 280:43–51
De Rivas J, Gutierrez-Ortiz R, Lopez-Fonseca J, Gonzalez-Velasco JI (2006) Analysis of the simultaneous catalytic combustion of chlorinated aliphatic pollutants and toluene over ceria-zirconia mixed oxides. App Catal A 314:54–63
Trovarelli A (1996) Catalytic properties of ceria and CeO2-containing materials. Catal Rev-Sci Eng 38:439–520
Koseoglu Y, Alan F, Tan M, Yilgin R, Oztiirk M (2012) Low temperature hydrothermal synthesis and characterization of Mn doped cobalt ferrite nanoparticles. Ceram Int 38:3625–3634
Liu Y, Wang Y, Zhou S, Lou S, Yuan L, Gao T, Wu X, Shi X, Wang K (2012) Synthesis of high saturation magnetization superparamagnetic Fe3O4 hollow microspheres for swift chromium removal. ACS Appl Mater Interfaces 4:4913–4920
Jovanovic S, Spreitzer M, Tramsek M, Trontelj Z, Suvorov D (2014) Effect of oleic acid concentration on the physicochemical properties of cobalt ferrite nanoparticles. J Phys Chem C 118:13844–13856
Luo, Y., Fu, D., Zhang, S., Yuan, Y., Zhai, S., Dong, H. Zhai, Temperature dependent coercivity and magnetization of light rare-earth Nd doped permalloy thin films. J. Magn. Magn. Mater. 374 (2015) 711–715.
Ayyappan S, Panneerselvam G, Antony MP, Philip J (2011) Structural stability of ZnFe2O4 nanoparticles under different annealing conditions. Mater Chem Phys 130:1300–1306
Acknowledgements
The authors would like to thank and appreciate the Ministry of Science & Research, Research Department of Sistan & Baluchestan University, as well as the Iranian National Petrochemical Company (INPC) for financial supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taherzadeh Lari, T., Mirzaei, A.A. & Atashi, H. Fischer–Tropsch Synthesis: Effects of Aging Time and Operating Temperatures on Solvothermally Prepared Nanocatalyst for Light Olefin Selectivity. Catal Lett 147, 1221–1234 (2017). https://doi.org/10.1007/s10562-017-2019-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2019-3