Abstract
Iron/nanoporous carbon catalysts (Fe/NPC) were used in the studies on limonene oxidation employing methanol as a solvent and aqueous hydrogen peroxide as an oxidizing agent. The nanoporous carbon support of the catalyst was produced from molasses, a sugar refinery waste product. To the best of our knowledge, there are no reports on the preparation of nanoporous carbon from molasses. The content of iron in the Fe/NPC catalysts studied included 0.68, 1.32 and 2.64 wt% Fe. The products of the limonene oxidation process were 1,2-epoxylimonene and its diol, carveol and perillyl alcohol, which are compounds with a large number of applications. The conversion of limonene reached 60 mol%. The “pure” NPC material was also active in the oxidation process. The utilized catalytic system is simple and cheap, and the use of environmentally-friendly H2O2 and the natural product limonene signify that this process can be considered as a “green process”. The results of the current research indicate that the utilization of molasses for the preparation of the carbonaceous catalysts active in the limonene oxidation is feasible, and these catalysts could be used in numerous industrial oxidation processes.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
During the last 10 years, since the pioneering work of Toda and others [1], the development of new carbon catalysts prepared from sugars, including glucose [2, 3] and sucrose [4] has been observed. For example, the esterification of free fatty acids with methanol using the sulfonated porous carbons produced from sugars resulted in biodiesel [4]. These materials were recyclable and environmentally friendly. Sugars are very low-cost starting materials, however, a cheaper, more economical and sustainable industrial waste product (sugar beet molasses) for the synthesis of carbon catalysts has been proposed [5]. According to the best of our knowledge, there is only one report concerning the utilization of molasses as the raw material for catalysts. Such catalysts were made by the incomplete carbonization of molasses followed by sulfonation. Molasses was used very rarely as a useful raw material. The other example of the use of molasses is for the production of an activated carbon for the adsorption of gases [6] and liquids [7].
Cyclic terpene hydrocarbons represent a group of attractive intermediates for broad applications in “green” organic processes. They are non-toxic and can be described as biodegradable compounds [8]. Nowadays, special attention is directed to R(+)-limonene the natural enantiomer obtainable from citrus products. Limonene is a colorless oil, sparingly soluble in water, and with a sweet orange smell. This terpene is widely used as flavors and fragrances in the food and cosmetic industry, and also in the production of refrigerant fluids, paints, agrochemicals and cleaning agents [9–12]. R(+)-limonene is commercially obtained from the biomass of orange and lemon peels, which are waste products obtained in large amounts during juice production in the citrus fruit industry. The main methods of extraction of R(+)-limonene from orange peels are the cold pressing method (mainly used in the citrus fruit industry) and simple distillation (the method mainly used in the laboratory). The large amount of limonene obtained from waste orange peels (about 70,000 tons per year world-wide) shows that this compound is a renewable, very cheap and easily available biointermediate for the synthesis of new important chemicals [13].
The limonene oxidation process is very complicated, because apart from the production of 1,2-epoxylimonene the formation of the following products is also observed: 1,2-epoxylimonene diol, carvone, carveol, perillyl alcohol, and in smaller amounts, 8,9-epoxylimonene, 8,9-epoxylimonene diol, diepoxide (1,2- and 8,9-) and its diol, perillal and perillyl acid [10, 11, 14–18]. These oxygenated derivatives of limonene are very valuable intermediates used in the production of flavors, perfumes, cosmetics, food additives, drugs and agrochemicals [11, 15] and also polymers—especially fragrant ones [19, 20]. Moreover, perillyl alcohol is an efficacious compound against the formation and progression of various cancers (pancreatic, mammary and liver tumors) and it is a chemopreventive agent for colon, skin and lung cancer [21, 22].
During the last 2 years we extensively studied the oxidation of limonene over the various titanium silicate catalysts: TS-1, TS-2, Ti-Beta, Ti-MCM-41, Ti-MWW and Ti-SBA-15. The studies showed that the main directions of transformations of limonene were: epoxidation of limonene to 1,2-epoxylimonene, hydration of 1,2-epoxylimonene to diol, hydroxylation of limonene to perillyl alcohol, hydroxylation of limonene to carveol and oxidation of limonene to carvone [14, 23, 24]. We also observed the same directions of the reactions when we examined the catalyst in the form of an activated carbon EuroPh supported Fe [25].
The current work examines the catalytic performance of the inexpensive Fe/nanoporous carbon catalysts (Fe/NPC) having different Fe content, which were obtained from molasses and nano-sized silica templates. It is environmentally friendly to exploit agro-industrial residues and to apply the following procedures: molasses and silica template mixing, pyrolysis of molasses, removal of the silica template followed by metal incorporation and thermal activation, resulting in the formation of effective catalysts for limonene oxidation. To the best of our knowledge, there are no reports on the preparation of nanoporous carbon from molasses and the utilization of such Fe/NPC catalysts obtained on the basis of this carbon in limonene oxidation. Moreover no any carbonaceous material has been used as catalyst in limonene oxidation. The utilization of hydrogen peroxide as the oxidizing agent is ecologically friendly in that the only product of the oxidant transformation is water. Moreover, the reaction with limonene can be performed at mild conditions and at atmospheric pressure. The proposed method of limonene epoxidation can be designated as a “green method” due to the utilization of natural products (molasses-based catalysts and limonene, which are renewable biointermediates) and also by the use of the environmentally-friendly hydrogen peroxide in the oxidation of limonene. This makes the proposed Fe/NPC catalyst an ecologically friendly, very easy and cheap catalytic system. The aim of this work was also to test the possibility of the separation and reuse of the catalysts used in the oxidation of limonene. In addition, the examinations of the activity of the “pure” NPC material in oxidation of limonene were performed.
2 Experimental
For the synthesis of the Fe/NPC catalysts, tetraethyl orthosilicate (TEOS) and iron(III) nitrate nonahydrate [Fe(NO3)3·9H2O] obtained from Sigma-Aldrich were used. Aqueous ammonia solution (25–28 wt%) and ethanol (96% purity) were acquired from Chempur. Analytical grade potassium hydroxide (KOH) was supplied from Eurochem BGD. Molasses was kindly provided by the National Sugar Company located in Kluczewo, Poland. In the oxidation of limonene the following raw materials were used: R(+)-limonene (97%, Sigma), aqueous hydrogen peroxide (60 wt%, Chempur), and methanol (analytical grade, Chempur).The synthesis of the Fe/NPC catalysts was performed in three steps: (I) SiO2 solid spheres synthesis, (II) nanoporous carbon (NPC) synthesis, and (III) NPC impregnation by iron nitrate. SiO2 solid spheres were prepared by a suitably modified previously reported procedure [26]; namely, deionized water, NH3(aq) and C2H5OH were added successively to TEOS in the following volume ratios −3:1:23:2, respectively. The resulting mixture was then stirred for 3 h at room temperature with a speed of 250 rpm. The white silica colloidal suspension that was obtained was centrifuged and washed three times with deionized water and followed with ethanol, and silica particles were obtained. The resulting solid powder was then dried overnight at room temperature.
Nanoporous carbon (NPC) was obtained using molasses and SiO2 solid spheres with the mass ratio of 1.22:1, respectively. The mixture was dried at a temperature of 80 °C during 2 h followed by a temperature of 160 °C during 16 h. The pyrolysis was performed in a tubular furnace at 750 °C for 2 h. In order to remove the silica templates, the product of the pyrolysis was stirred in an aqueous HF solution (7 wt%) for 24 h, followed by filtration, washing with deionized water, and drying at 200 °C for 12 h. Finally, a black colored NPC was obtained. This method of NPC production from molasses was not described up to now, and two of the co-authors of this manuscript are inventors of the pending patent [27].
In the next stage, the obtained NPC was mixed with an aqueous solution of Fe(NO3)3·9H2O and left for 1 h with magnetic stirring. The resulting suspension was treated by sonication for 1 h, and the excess of water was evaporated at 100 °C for 2 h. The sample was then calcinated at 550 °C in a tubular oven with air flow. In this procedure, Fe-impregnated nanoporous carbon catalysts containing 0.68, 1.32 and 2.64 wt% of Fe were obtained. These catalysts were labelled as mFe/NPC where “m” refers to the mass percentage concentration of Fe. For example, the sample labelled as 0.68Fe/NPC describes the sample that was doped with 0.68 wt% Fe.
The XRD patterns were recorded using X’Pert PRO diffractometer with CuKα radiation. The characterization of the porous texture of the produced catalysts was performed by the adsorption–desorption of nitrogen at the temperature of 77 K and at subatmospheric pressures using the Quadrasorb automatic adsorption system (Quantachrome Instruments). The samples were degassed for 14 h under high vacuum and at 250 °C (final pressure 10−4 Torr). The specific surface areas were calculated by the multipoint Brunauer–Emmett–Teller method (SBET). The relative pressure p/po range of nitrogen isotherms (0.08–0.24) was applied. Within this partial pressure region the linearity of 1/(V[(po/p) − 1]) versus p/po plot was satisfactory, where po = 7622 Torr and V is the volume of gas adsorbed at p/po. The total pore volume, Vp, was estimated at p/po equal to 0.99.
The iron content of the produced catalysts was determined by the X-ray fluorescence spectroscopy method.
Ultra-high resolution field emission scanning electron microscope equipped with energy dispersive spectroscopy system (EDS) using a UHR FE-SEM Hitachi SU8020 was used to characterise the nature of the catalysts and iron content on the catalysts surface.
The epoxidation of limonene was performed at a temperature of 70 °C and for the reaction times from 0.5 to 24 or 48 h. The other parameters were as follows: the molar ratio of limonene/H2O2 = 1:2, methanol concentration 95 wt% and the catalyst content in the reaction mixture of 2.45 wt%. The process was carried out in a glass reactor with the capacity of 25 cm3, equipped with a reflux condenser, a thermometer and a magnetic stirrer. The raw materials were placed into the glass reactor in the following order: catalyst, limonene, methanol and 60 wt% aqueous solution of hydrogen peroxide. The temperature of 70 °C was achieved with a silicon oil bath. The progress of the reaction was examined after the following reaction times: 30 min and 1, 1.5, 2, 2.5, 3, and 24 h, and in some cases, 48 h. Samples taken at different reaction times were analysed by a GC-method on a Focus apparatus equipped with a flame-ionization detector and fitted with the Restek Rtx-WAX capillary column filled with polyethylene glycol. The parameters of the GC-method were as follows: helium pressure of 50 kPa, sensitivity of 100, the temperature of the sample chamber 200 °C, the detector temperature of 250 °C, and the temperature of the thermostat was increased according to the following program: isothermally at the temperature of 60 °C for 2 min, an increase to the temperature of 240 °C at the rate of 15 °C/min, isothermally at the temperature of 240 °C for 4 min, and at the last stage cooling to the temperature of 60 °C. The products of limonene epoxidation were also qualitatively identified by GC-MS method.
The hydrogen peroxide conversion was measured by the iodometric titration method. The mass balance for the each sample taken after the appropriate reaction time was calculated. On the basis of this mass balance the main functions describing the process of limonene epoxidation were calculated: the selectivities of the appropriate products in relation to limonene (L)—Sproduct/L, the conversion of limonene—CL, the conversion of hydrogen peroxide—\({{\text{C}}_{{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}}}\) and the selectivity of transformation to organic compounds in relation to hydrogen peroxide consumed (the efficiency of hydrogen peroxide conversion)—\({{\text{S}}_{\text{org}\text{. comp}\text{./}{{\text{C}}_{{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}}}}}\). These main functions were calculated on the basis of the following equations:
3 Results and Discussions
Figure 1 shows the SEM images of NPC (support) and the 2.64Fe/NPC catalyst. The unusual structure of the support was achieved due to SiO2 solid spheres that were removed after calcination. During incorporation of iron this structure was not damaged. The surface content of Fe estimated on the basis of EDS results were: 0.8, 1.6 and 3.2 wt% for 0.68Fe/NPC, 1.32Fe/NPC and 2.64Fe/NPC catalysts, respectively.
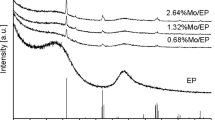
On the basis of the XRD investigations it was found that Fe3O4 was formed during calcination of the Fe/NPC catalysts. Fe3O4 is a well-known ferromagnetic oxide with equal mole ratios of Fe(II) and Fe(III) oxides.
The results of nitrogen sorption measurements shown in Fig. 2 revealed rather unusual features in the isotherms for the support (NPC) and the 0.68Fe/NPC, 1.32Fe/NPC and 2.64Fe/NPC catalysts. The overall shape of the nanoporous carbon isotherm is type IV characteristic for mesoporous structures but no plateau over a range of high p/p0 is observed. The unlimited uptake indicates incomplete pore filling. The macropores can be responsible for such shapes of isotherms. The hysteresis loop represents typical H3 behaviour but hysteresis is observed in the entire pressure region. Such a phenomenon was observed for various adsorbents [28], but according to our knowledge, never for nitrogen at 77 K. The desorption branch also contains a steep region (typical for type H3 hysteresis), however the hysteresis loop does not close but becomes narrower.
The swelling of non-rigid pores or the irreversible uptake of nitrogen may be the reason for low-pressure hysteresis [29]. Irreversible uptake occurs in pores of about the same width as that of the nitrogen molecule. An interpretation of sorption isotherms with low-pressure hysteresis is not simple and an accurate pore size distribution analysis is impossible.
The SBET of NPC, 0.68Fe/NPC, 1.32Fe/NPC, 2.64Fe/NPC was found to be equal to 386, 440, 424, 377 m2/g and Vtot equal to 0.391, 0.338, 0.322, 0.289 cm3/g, respectively.
The studies on the limonene oxidation over the 0.68Fe/NPC catalyst with the 60 wt% hydrogen peroxide as the oxidizing agent are presented in Figs. 3 and 4.
The influence of the reaction time on the conversion of limonene (CL) and the selectivity of the transformation to organic compounds in relation to hydrogen peroxide consumed (\({{\text{S}}_{\text{org}\text{. comp}\text{./}{{\text{C}}_{{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}}}}}\)); graphs relate to hydrogen peroxide over the 0.68Fe/NPC catalyst
Figure 3 shows that the main product of the process of limonene oxidation over the 0.68Fe/NPC catalyst is not 1,2-epoxylimonene but the product of allylic oxidation at the 6-position in the limonene molecule—carveol (Fig. 5). This reaction is also named as the hydroxylation at the 6-position or cyclic allylic hydrogen abstraction [14, 30].
Carveol was stable at the condition at which the reaction was performed and did not undergo the oxidative dehydrogenation to carvone. Its selectivity was almost unchanged during the prolongation of the reaction time and amounted to 97–99 mol% (for the reaction time longer than 0.5 h). The formation of the product of the epoxidation of the unsaturated bond at the 1,2-position (1,2-epoxylimonene) was also observed for the 0.68Fe/NPC catalyst [14, 30] (Fig. 6).
However, 1,2-epoxylimonene was formed with a very small selectivity of about 1–3 mol%. 1,2-epoxylimonene was a stable compound under the examined conditions and did not undergo further conversion to 1,2-epoxylimonene diol. The product of the allylic oxidation at the 7-position in the limonene molecule (perillyl alcohol) was not detected in the reaction mixtures. Similar to the note mentioned above, this reaction is also named as the hydroxylation at the 7-position or acyclic allylic hydrogen abstraction [14, 27] (Fig. 7).
The oxidation of limonene over the 0.68Fe/NPC catalyst underwent with a very low conversion of limonene—from 1 to 7 mol% and at a very low effective conversion of hydrogen peroxide to organic compounds—from about 1 to 4 mol% (Fig. 4). The values of the last functions show that the ineffective decomposition of hydrogen peroxide over the NPC underwent very fast, and faster than the reactions at the active centers of Fe. Thus, the conversion of limonene was very low.
The studies on the limonene epoxidation over the 1.32Fe/NPC with the 60 wt% hydrogen peroxide as the oxidizing agent are presented in Figs. 8 and 9.
The influence of the reaction time on the selectivities of the main products of limonene epoxidation with hydrogen peroxide over the 1.32Fe/NPC catalyst (abbreviations are given in Fig. 3), the right Y axis presents the values of the selectivity of carveol and perillyl alcohol
The influence of the reaction time on the conversion of limonene (CL) and the selectivity of transformation to organic compounds in relation to hydrogen peroxide consumed (\({{\text{S}}_{\text{org}\text{. comp}\text{./}{{\text{C}}_{{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}}}}}\)); graphs relate to hydrogen peroxide over the 1.32Fe/NPC catalyst
Figure 8 shows that for the reaction time of 1–2 h the main product of the process of limonene oxidation was perillyl alcohol, which was formed with the selectivity of 49–56 mol%. For longer reaction times, the main product of this process was 1,2-epoxylimonene but it was detected in the reaction mixtures in only small amounts. It was caused by the secondary reaction in which this epoxide compound participated—hydration of the epoxide ring and formation of 1,2-epoxylimonene diol. The selectivity of 1,2-epoxylimonene diol changed from 63 to 74 mol% for the reaction time in the range of 3–24 h, and at the same range of the reaction time, selectivity of 1,2-epoxylimonene amounted to about 1 mol%. For shorter reaction times the selectivity of 1,2-epoxylimonene was nearly 0 mol%. The formation of carveol was observed in small amounts with the prolongation of the reaction time (with a selectivity of about 3–9 mol%). Carveol was a stable compound and did not undergo oxidative dehydrogenation to carvone. These results show that for short reaction times the main direction of this process was allylic oxidation at the 7-position (perillyl alcohol formation); however, longer reaction times were preferential to the epoxidation at the 1,2-position in the limonene molecule and formation of 1,2-epoxylimonene, which very quickly underwent hydration to 1,2-epoxylimonene diol.
During the studies on limonene epoxidation over the 1.32Fe/NPC catalyst, it was noticed that the conversion of limonene was considerably higher than for the 0.68Fe/NPC catalyst and changed from 0 to 41 mol% for the reaction time from 0.5 to 24 h (Fig. 9). Also the selectivity of the transformation to organic compounds in relation to hydrogen peroxide consumed was higher than for 0.68Fe/NPC catalyst and amounted to 0–20 mol%.
The studies on the limonene epoxidation over the 2.64Fe/NPC catalyst with the 60 wt% hydrogen peroxide as the oxidizing agent are presented in Figs. 10 and 11.
The influence of the reaction time on the selectivities of the main products of limonene epoxidation with hydrogen peroxide over the 2.64Fe/NPC catalyst (abbreviations are given in Fig. 3), the right Y axis presents the values of the selectivity of 1,2-epoxylimonene
The influence of the reaction time on the conversion of limonene (CL) and the selectivity of transformation to organic compounds in relation to hydrogen peroxide consumed (\({{\text{S}}_{\text{org}\text{. comp}\text{./}{{\text{C}}_{{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}}}}}\)); graphs relate to hydrogen peroxide over the 2.64Fe/NPC catalyst
Figure 10 shows that the main product of the process of limonene oxidation over the 2.64Fe/NPC catalyst, independent of the reaction time after 2 h, is perillyl alcohol. The selectivity of this compound changed from 77 mol% (for the reaction time of 1 h) to about 61–62 mol% for longer reaction times. The second product, which was detected in the reaction mixtures, was 1,2-epoxylimonene diol. It was formed with the selectivity from 23 mol% (for the reaction time of 1 h) to 36 mol% (for reaction time of 2.5–48 h). 1,2-epoxylimonene was present in the reaction mixtures in a small amount, with a selectivity of about 1–2 mol%, independent of the reaction time. The same as for the studies over the 1.32Fe/NPC catalyst, the epoxide compound very easily underwent hydration to diol. The conversion of limonene over 2.64Fe/NPC was considerably higher than for the 0.68Fe/NPC and 1.32Fe/NPC catalysts (Fig. 11). This function changed from 13.1 mol% (reaction time 1 h) to 60 mol% (reaction time 48 h). Also the efficiency of hydrogen peroxide conversion was higher and ranged from 41.2 mol% (reaction time 1 h) to 48 mol% (reaction time 48 h).
The reaction with hydrogen peroxide that does not use a dissolved Fe2+ homogeneous catalyst is defined as a Fenton-like reaction. This reaction can be catalyzed by heterogeneous catalysts including Fe3+, native or added as iron oxides, or by certain transition metals. Iron oxides are capable of decomposing hydrogen peroxide. Hydrogen peroxide and Fe3+ undergo a redox cycle in which ·OH radicals are produced. Taking into account the results presented for the 0.68Fe/NPC, 1.32Fe/NPC and 2.64Fe/NPC catalysts, and the analysis of the literature data [31–36], the paths for the transformation of hydrogen peroxide in the presence Fe/NPC catalysts presented in Fig. 12 can be proposed.
During the heterogeneous Fenton-like reactions catalyzed by Fe/NPC catalysts, Fe(III) atoms are reduced to Fe(II) by hydrogen peroxide and by ·OOH radicals formed in the reaction of H2O2 with Fe(III). Fe(II) atoms then react with H2O2 and in this reaction Fe(III) is formed and the ·OH radical is released as a reactive species which can participate in the oxidation of organic compounds. On the other hand, the reaction of Fe(II) atoms with H2O2 might lead to Fe(IV)—feryl—species. This active species can directly oxidize organic substrates and during this oxidation Fe(II) is formed 32.
Taking into account the various active species, which are formed in the Fenton-like oxidations, the possible mechanism of caveol formation presented in Fig. 13 can be proposed.
For the formation of perillyl alcohol a similar mechanism can be proposed (Fig. 14).
In the formation of carveol and perillyl alcohol, the ·OOH radicals can also participate. As a result of the transformation of this radical an ·OH radical is also formed according to the reaction.
The formation of 1,2-epoxylimonene can be explained by proposing that the ·OOH radicals or ferryl species [–Fe(IV) = O] oxidize the unsaturated bond of limonene at the 1,2-position by the addition of oxygen to this bond (Fig. 15).
In the studies on the oxidation of limonene with hydrogen peroxide over the Fe/NPC catalysts, the possibility of activity of the support (the “pure” NPC obtained from molasses) in this reaction should also be taken into account. Therefore, the oxidation of limonene over the NPC obtained from molasses was also performed. The results of these studies are presented in Figs. 16 and 17.
The influence of the reaction time on the selectivities of the main products of limonene epoxidation with hydrogen peroxide over the NPC obtained from molasses (abbreviations are given in Fig. 3)
The influence of the reaction time on the conversion of limonene (CL) and the selectivity of transformation to organic compounds in relation to hydrogen peroxide consumed (\({{\text{S}}_{\text{org}\text{. comp}\text{./}{{\text{C}}_{{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}}}}}\)); graphs relate to hydrogen peroxide over the NPC catalyst obtained from molasses
These figures show that the NPC material obtained from molasses was active in the oxidation of limonene with hydrogen peroxide, but the first product of this process (carveol) was obtained only after the reaction time of 3 h. For the Fe/NPC catalysts, independent of the Fe content, the first products were detected after the reaction time of 1 h. In case of the NPC material the prolongation of the reaction time from 5 to 48 h causes formation not only of carveol but also of carvone (this product was not observed during studies on the Fe/NPC catalysts) and perillyl alcohol. On the other hand, the formation of 1,2-epoxylimonene and its diol was not observed. It can be shown that the main participant in the production of 1,2-epoxide is the Fe(IV), ferryl, species, which is not formed during the oxidation over the “pure” NPC material. For the NPC material the conversion of limonene after the reaction time of 48 h amounted to about 30 mol% and was higher than for the 0.68Fe/NPC catalyst but lower than for 1.32Fe/NPC and 2.64Fe/NPC catalysts. The efficiency of hydrogen peroxide conversion for the oxidation over the NPC material for the reaction time of 48 h amounted to about 43 mol% and was higher than for 0.68Fe/NPC and 1.32Fe/NPC catalysts but similar to the 2.64Fe/NPC catalyst.
Taking into account the literature data [37–40], the mechanism of the ·OH and ·OOH radicals formation over the NPC material obtained from molasses for the active carbon (AC) can be proposed.
The ·OH and ·OOH radical species are formed by the electron-transfer reaction similar to the Fenton mechanism, with AC and AC+ as the reduced and oxidized catalyst states, respectively. The recombination of free radical species (·OH and ·OOH) in the liquid phase or onto the active carbon surface will produce water and oxygen according to the reaction:
According to this mechanism of radical species ·OH and ·OOH production over NPC material the formation of carvone as the product can be explained (Fig. 18).
The studies on the limonene epoxidation over the reused Fe/NPC catalysts and with hydrogen peroxide showed that 0.68Fe/NPC catalyst was still active in this process. After the reaction time of 3 h the conversion of limonene was 7 mol% and after 24 h, 15 mol%. The efficiency of hydrogen peroxide conversion for 3 h had the same values as conversion of limonene for the appropriate reaction time. The only product of the reaction was perillyl alcohol. In comparison with the first utilization (the first run) of this catalyst, the conversion of limonene increased, especially for the reaction time of 24 h (from 5 to 15 mol%). Also the increase in values of the efficiency of hydrogen peroxide conversion was observed (for the reaction time 24 h from 3 to 14 mol%). The direction of the oxidation reaction also changed from allylic oxidation at the 6-positon to allylic oxidation at the 7-position.
The studies over the reused 1.32Fe/NPC catalyst and hydrogen peroxide as the oxidizing agent showed a considerable decrease in the activity of the reused catalyst. The conversion of limonene after 3 h amounted to 2 mol% (in the first run it amounted to 21 mol%) and after the reaction time of 24 h, 5 mol% (in the first run it amounted to 41 mol%). The efficiency of hydrogen peroxide conversion for the reaction time 3 h reached 2 mol% (in the first run it amounted to 10 mol%) and for the reaction time 24 h, 6 mol% (in the first run it amounted to 20 mol%). For the studies with the reused 1.32Fe/NPC catalyst only one product was observed—perillyl alcohol. In the first run a different direction of the oxidation was observed—the epoxidation of the unsaturated bond at the 1,2-position.
The studies over the reused 2.64Fe/NPC catalyst and hydrogen peroxide as the oxidizing agent also showed a considerable decrease in the activity of the reused catalyst. The conversion of limonene after 3 h amounted to 4 mol% (in the first run it amounted to 25 mol%) and after the reaction time of 24 h, 28 mol% (in the first run it amounted to 58 mol%). The efficiency of hydrogen peroxide conversion for the reaction time of 3 h reached 16 mol% (in the first run it amounted to 46 mol%) and for the reaction time of 24 h, 24 mol% (in the first run it amounted to 48 mol%). In these studies the only product was also perillyl alcohol. This direction of reaction was the same as in the first run.
To summarize, the reused Fe/NPC catalysts, when still active in the oxidation of limonene, directed the reaction to perillyl alcohol independent of the used sample of the catalyst. The decrease in the activity of the reused Fe/NPC catalysts was also confirmed by the textural studies. It was observed during textural studies that not only SBET but also Vtot decreased to the following values, respectively: 40–46 and 0.16–018 cm3/g. The XRD data of the used catalysts also showed a decrease in the amount of Fe3O4 but the amount of the catalysts was not enough for quantitative analyses.
A comparison of the results obtained in this work with our previous results obtained for the catalyst in the form of the activated carbon EuroPh (Ep) with the same content of Fe and in the same testing reaction [25] shows that Fe/NPC catalysts are more active than FeEP catalysts. This disparity is especially noticeable for the first two Fe contents (0.68 and 1.32 wt%), because for the Fe/NPC catalysts the first products were formed after 1 h, whereas for the FeEP catalysts only after 5 h. For the 0.68/NPC catalyst after 48 h the conversion of limonene was considerably lower (7 mol%) than for 0.68Ep (82 mol%) but it was possible to obtain very high perillyl alcohol selectivity (97–99 mol%), which is very beneficial when taking into account the applications of this compound. For the 0.68Ep catalyst three products were obtained: 1,2-epoxylimonene diol, carvone and perillyl alcohol and the selectivity of perillyl alcohol reached the maximal value 53 mol% after 5 h. For the 0.68/NPC catalyst also very high effective decomposition of H2O2 was observed (96 mol%) in comparison with the FeEp catalyst (3 mol%).
For the 1.32/NPC catalyst after 24 h the conversion of limonene was higher (41 mol%) than for the 1.32Ep catalyst (30 mol%). For the 1.32Ep catalyst, formation of 1,2-epoxylimonene diol, carvone and perillyl alcohol were observed, but the main product was perillyl alcohol (selectivity 42–45 mol%). For the 1.32/NPC catalyst the following products were observed: 1,2-epoxylimonene diol, carveol and perillyl alcohol; only one product was different—carveol—oxidation still proceeded at the 6-position in the limonene molecule but slower and in the direction of the alcohol, not the ketone. With this catalyst and for the reaction time of up to 2 h the main product was perillyl alcohol, the same as for the 1.32Ep catalyst, but for longer reaction times the main product started to be 1,2-epoxylimonene diol (selectivity 74 mol% for 24 h).
For the Fe content of 2.64 wt% the first products were obtained with both Fe/NPC and FeEp catalysts after 1h. Carvone or carveol formation was not observed. For the 2.64EP catalyst the main product was 1,2-epoxylimonene diol (from 71 to 63 mol%) and for the 2.64/NPC catalyst perillyl alcohol (from 77 to 61 mol%) but when taking into account the applications of these two compounds perillyl alcohol formation is more preferable. With the 2.64Ep catalyst limonene conversion after 48 h was 100 mol% and for 2.64/NPC 60 mol%.
4 Conclusions
The Fe/NPC and “pure” NPC were active catalysts in limonene oxidation. It is possible to select such technological parameters at which one of the products (perillyl alcohol) is obtained with high selectivity. Limonene oxidation process over the catalyst obtained from molasses (an agro-industrial waste product) can be described as eco-friendly as it uses limonene (from orange peels) and environmentally-friendly hydrogen peroxide under mild conditions. It was found that the easy preparation and long-term stability under the oxidation conditions make the Fe/NPC and “pure” NPC very useful catalysts for further applications in the oxidation processes in the organic industry. Examples are the epoxidation of allylic alcohols such as allyl alcohol, crotyl alcohol, methallyl alcohol, epoxidation of allyl chloride or hydroxylation of aromatic compounds, especially phenol to hydroquinone and pyrocatechol.
New directions in developing the process of limonene oxidation can be: utilization of other solvents that can help to increase selectivities of other products (for example, 1,2-epoxylimonene) or application of other oxidizing agents, for example, t-butyl hydroperoxide, in this process. Taking into account that molasses and orange peels are a part of biomass and thus renewable sources, and the applications of such raw materials is in tune with the latest trends in the organic industry, the process of limonene oxidation has a good chance of implementation on an industrial scale in the future.
Abbreviations
- NPC:
-
Nanoporous carbon
- TEOS:
-
Tetraethyl orthosilicate
- Ep:
-
Commercial activated carbon EuroPh
- L:
-
Limonene
- CL :
-
Conversion of limonene
- Sproduct/L :
-
Selectivity of the appropriate products in relation to limonene
- \({{\text{C}}_{{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}}}\) :
-
Conversion of hydrogen peroxide
- \({{\text{S}}_{\text{org}\text{. comp}\text{./}{{\text{C}}_{{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}}}}}\) :
-
Selectivity of transformation to organic compounds in relation to hydrogen peroxide consumed (the efficiency of hydrogen peroxide conversion)
- EP:
-
1,2-Epoxylimonene
- EPDIOL:
-
1,2-Epoxylimonene diol
- PA:
-
Perillyl alcohol
- Fe/NPC:
-
Fe/nanoporous carbon catalysts
References
Toda M, Takagaki A, Okamura M, Kondo JN, Hayashi S, Domen K, Hara M (2005) Nature 438:178
Zong M, Duan Z, Lou W, Smith TJ, Wu H (2007) Green Chem 9:434
Takagaki A, Toda M, Okamura M, Kondo JN, Hayashi S, Domen K, Hara M (2006) Catal Today 116:157
Lou W, Zong M, Duan Z (2008) Bioresour Technol 99:8752
Samori C, Torri C, Fabbri D, Falini G, Faraloni C, Galletti P, Spera S, Tagliavini E, Torzillo G (2012) ChemSusChem 5:1501
Sreńscek-Nazzal J, Kamińska W, Michalkiewicz B, Koren ZC (2013) Ind Crop Prod 47:153
Legrouri K, Khouya E, Ezzine M, Hannache H, Denoyel R, Pallier R, Naslain R (2005) J Hazard Mater 118:259
Bahr M, Mulhaupt R (2012) Green Chem 14:1447
Ciriminna R, Lomeli-Rodrigues M, Demm Cara P, Lopez-Sanches JA, Pagliaro M (2014) Chem Commun 50:15288
Pena A, Veiga S, Sapelli M, Martinez N, Marquez V, Dellacassa E, Bussi J (2012) React Kinet Mech Catal 107:263
Santa AM, Vergara JC, Palacio LA, Echavarria A (2008) Catal Today 133–135:80
Monteiro JLF, Veloso CO (2004) Top Catal 27:169
Firdaus M, Meier AR (2013) Green Chem. 15:370
Wróblewska A (2014) Molecules 19:19908
Corma A, Iborra S, Velty S (2007) Chem Rev 107:2411
Cagnoli MV, Casuscelli SG, Alvarez AM, Bengoa JF, Gallegos NG, Samaniego NM, Crivello ME, Ghione GE, Perez CF, Herrero ER (2005) Appl Catal A 287:227
Robles-Dutenhefner PA, Brandao BBNS, de Sousa LF, Gasevskaya EV (2011) Appl Catal A 399:172
Bonon AJ, Mandelli D, Kholdeeva OA, Barmatova MV, Kozlov YN, Shulpin GB (2009) Appl Catal A 365:96
Byrne CM, Allen SD, Lobkovsky EB, Coates GW (2004) J Am Chem Soc 126:11404
Wilborn PA, Chu F, Tang C (2013) Macromol Rapid Commun 34:8
Gupta A, Straton SP, Myrdal PB (2005) J Pharm Biomed Anal 37:447
Gupta A, Myrdal PB (2004) Int J Pharm 269:373
Wróblewska A, Makuch E (2015) Pol J Chem Technol 4:82
Wróblewska A, Makuch E, Miądlicki P (2016) Catal Today 268:121
Młodzik J, Wróblewska A, Makuch E, Wróbel RJ (2016) Catal Today 268:111
Fang X, Chen C, Liu Z, Liu P, Zheng N (2011) Nanoscale 3:1632
Michalkiewicz B, Majewska J, Młodzik J, Michalkiewicz K (2014) Sposób otrzymywania nanoporowatych materiałów węglowych, Polish Patent Pending P 406919
Huang Y, Zheng X, Duan J, Liu W, Zhou L, Wang C, Wen L, Zhao J, Li D (2014) Dalton Trans 43:6811
Lowell S, Shields J, Thomas M, Thommes M (2004) Characterization of porous solids and powders. Kluwer, Dordrecht
Rothenberg G, Yatziv T, Sasson Y (1998) Tetrahedron 54:593
Banerjee S, Santra S (2013) J Catal Article ID 910489 1–5
Pham AL-T, Doyle FM, Sedlak DL (2012) Water Res 46:6454
Gan S, Venny HK, Ng HK (2012) Chem Eng J 213:295
Yap CL, Gan S, Ng HK (2011) Chemosphere 83:1414
Gonzales-Olmos R, Holzer F, Kopinke FD, Georgi A (2011) Appl Catal A 398:44
Traylor TG, Tsuchiya S, Byan Y-S, Kim C (1993) J Am Chem Soc 115:2775
Bach A, Semiat R (2011) Desalination 273:57
Georgi A, Kopinke FD (2005) Appl Catal B 58:9
Rey A, Zazo JA, Casas JA, Bahamonde A, Rodrigues JJ (2011) Appl Catal A 402:146
Aguinaco A, Pocostales JP, Garcia-Araya JF, Beltran FJ (2011) J Chem Technol Biotechnol 86:595
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wróblewska, A., Makuch, E., Młodzik, J. et al. Fe/Nanoporous Carbon Catalysts Obtained from Molasses for the Limonene Oxidation Process. Catal Lett 147, 150–160 (2017). https://doi.org/10.1007/s10562-016-1910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1910-7