Abstract
A new silica bonded N-(propylcarbamoyl)sulfamic acid (SBPCSA) catalyst has been prepared for the highly efficient synthesis of a series of acrylonitrile derivatives via solvent-free facile Knoevenagel condensation between differently substituted heterocyclic/aromatic aldehydes 1 (a–o) and 2-thiopheneacetonitrile (2). The catalyst was characterized by FT-IR, SEM-EDX and XRD techniques. The thermal stability of the catalyst was evaluated with TGA and DT analysis. The remarkable features of the present protocol are solvent free synthesis, recyclability of the catalyst, mild reaction conditions, shorter reaction profile, excellent yield of products with applicability to broader substrate scope (electron rich and electron deficient) and exclusive formation of E-isomer of the product. DFT calculations also revealed that E-isomer of compound 3f is stabilized by 12.53 kcal mol−1 more than the Z-isomer.
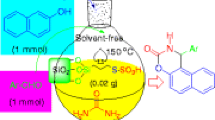
Graphical Abstract
Efficient and economical synthesis of acrylonitrile derivatives.
















Similar content being viewed by others
References
Trost BM (1991) Science 254:1471
Parveen M, Azaz S, Malla AM, Ahmad F, Silva PSPD, Silva MR (2015) New J Chem 39:469
Shylesh S, Schünemann V, Thiel WR (2010) Angew Chem Int Ed 49:3428
Parveen M, Azaz S, Malla AM, Ahmad F, Ahmad M, Gupta M (2016) RSC Adv 6:148
Clark JH (2002) Acc Chem Res 35:791
Princy G, Satya P (2014) Catal Today 236:153
Parveen M, Ahmad F, Malla AM, Alam M, Lee DU (2014) Catal Lett 144:2091
Parveen M, Ahmad F, Malla AM, Azaz S (2015) New J Chem 39:2028
Wang L, Zhao W, Tan W (2008) Nano Res 1:99
Gupta P, Paul S (2011) Green Chem 13:2365
Gupta P, Paul S (2014) Curr Catal 3:53
Gupta P, Paul S (2012) J Mol Catal A Chem 352:75
Gupta P, Kour M, Paul S, Clark JH (2014) RSC Adv 4:7461
Gupta P, Kumar V, Paul S (2010) J Braz Chem Soc 21:349
Zolfigol MA, Ayazi-Nasrabadi R, Baghery S (2016) Appl Organomet Chem 30:273
Knoevenagel E (1898) Ber Dtsch Chem Ges 31:2585
Bigi F, Chesini L, Maggi RR, Sartori G (1999) J Org Chem 64:1033
Yu N, Aramini JM, Germann MW, Huang Z (2000) Tetrahedron Lett 41:6993
Parveen M, Malla AM, Alam M, Ahmad M, Rafiq S (2014) New J Chem 38:1655
Gawande MB, Jayaram RV (2006) Catal Commun 7:931
Mukarram SMJ, Khan RAR, Yadav RP (2007) US Pat 0004935
Madivada LR, Anumala RR, Gilla G, Alla S, Charagondla K, Kagga M, Bhattacharya A, Bandichhor R (2009) Org Process Res Dev 13:1190
Beutler U, Fuenfschilling PC, Steinkemper A (2007) Org Process Res Dev 11:470
Tietze LF, Beifuss U (1991) In: Trost BM, Fleming I (eds) Comprehensive organic synthesis, vol 2. Pergamon Press, Oxford, p 341
Carta A, Palomba M, Boatto G, Busonera B, Murreddu M, Loddo R (2004) II Farmaco 59:637
Quiroga J, Cobo D, Insuasty B, Abonia R, Nogueras M, Cobo J, Vasquez Y, Gupta M, Derita M, Zacchino S (2007) Arch Pharm 340:603
Hranjec M, Pavlovic G, Marjanovic M, Kralj M, Zamola GK (2010) Eur J Med Chem 45:2405
Saczewski F, Stencel A, Bienczak AM, Langowska KA, Michaelis M, Werel W, Halasa R, Reszka P, Bednarski P (2008) Eur J Med Chem 43:1847
Sanna P, Carta A, Nikookar MER (2000) Eur J Med Chem 35:535
Biradar JS, Sasidhar BS (2011) Eur J Med Chem 46:6112
Litina EPDH, Litinas K, Geromichalos G (2014) Molecules 19:9655
Sanna P, Carta A, Gherardini L, Nikookar MER (2002) Farmaco II 57:79
Glazer EA, Chappel LR (1982) J Med Chem 25:766
Ali A, Bliese M, Rasmussen JM, Sargent RM, Saubern S, Sawutz DG, Wilkie JS, Winkler DA, Winzenberg KN, Woodgate RCJ (2007) Bioorg Med Chem Lett 17:993
Lieber S, Scheer F, Meissner W, Naruhn S, Adhikary T, Brüsselbach SM, Diederich WE, Muller R (2012) J Med Chem 55:2858
Janssen PAJ, Lewi PJ, Arnold E, Daeyaert F, Jonge M, Heeres J, Luc Koymans MH, Vinkers HM, Guillemont J, Pasquier E, Kukla M, Ludovici D, Andries K, Bethune M, Pauwels R, Das K, Clark AD Jr, Frenkel YV, Hughes SH, Medaer IB, Knaep FD, Bohets H, Clerck FD, Lampo A, Williams P, Stoffels P (1901) J Med Chem 48:1901
Wolfgang K, Ulrich S (2007) Modern crop protection compounds, vol 445. Wiley, New York
Chircop M, Perera S, Mariana A, Lau H, Ma MPC, Gilbert J, Jones NC, Gordon CP, Young KA, Morokoff A, Sakoff J, O’Brien TJ, McCluskey A, Robinson PJ (2011) Mol Cancer Ther 10:1553
Yi LW, Hai XQ, Xiang MY, Min LY, Li DN, Peng GD (2001) J Org Chem 625:128
El-Tammany S, Raulfs FW, Hopf H (1983) Angew Chem Int Ed Engl 22:633
Masllorens J, Manas MM, Quintana AP, Pleixats R, Roglans A (2002) Synthesis 13:1903
Thirupathi G, Venkatanarayana M, Dubey PK, Kumari YB (2012) Der Pharm Chem 4:1897
Tarleton M, Gilbert J, Sakoff JA, McCluskey A (2012) Eur J Med Chem 57:65
Biradar JS, Sasidhar BS (2011) Eur J Med Chem 46:6112
Karmakar B, Nayak A, Banerji J (2012) Tetrahedron Lett 53:5004
Abaee MS, Mojtahedi MM, Zahedi MM, Khanalizadeh G (2006) ARKIVOC 15:48
Yadav JS, Rao PP, Sreenu D, Rao RS, Kumar VN, Nagaiah K, Prasad AR (2005) Tetrahedron Lett 4:7249
Boullet FT, Foucaud A (1982) Tetrahedron Lett 23:4927
Morrison DW, Forbesa DC, Davis JH (2001) Tetrahedron Lett 42:6053
Harjani JR, Nara SJ, Salunkhe MM (2002) Tetrahedron Lett 43:1127
Tahmassebi D, Wilson LJA, Kieser JM (2009) Synth Commun 39:2605
Mulla SAR, Sudalai A, Pathan MY, Siddique SA, Inamdar SM, Chavan SS, Reddy RS (2012) RSC Adv 2:3525
Nemati F, Heravi MM, Rad RS (2012) Chin J Catal 33:1825
Mukhopadhyay C, Ray S (2011) Catal Commun 12:1496
Malla AM, Parveen M, Ahmad F, Azaz S, Alam M (2015) RSC Adv 5:19552
Parveen M, Ahmad F, Malla AM, Azaz S, Silva MR, Silva PSP (2015) RSC Adv 5:52330
Vafaeezadeh M, Hashemi MM, Fard MS (2012) Catal Commun 26:54
Ilczyszyn MM, Ilczyszyn M (2003) J Raman Spectrosc 34:693
Elsabawy KM (2014) Int J Pharm Res Health Sci 2:70
Maleki A, Alrezvani Z, Maleki S (2015) Catal Commun 69:29
Niknam K, Saberi D (2009) Tetrahedron Lett 50:5210
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Acknowledgments
Shaista Azaz, thanks the Chairman, Department of Chemistry, AMU, Aligarh, for providing the necessary research facilities, University Sophisticated Instrumentation Facility (USIF), AMU, Aligarh and Sophisticated Analytical Instrumentation Facility (SAIF), Chandigarh, is credited for spectral analysis. UGC is also gratefully acknowledged for research fellowship to S. Azaz and F. Ahmad.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parveen, M., Azaz, S., Ahmad, F. et al. Silica Bonded N-(Propylcarbamoyl)sulfamic acid (SBPCSA) Mediated Expeditious Approach to C–C Bond Formation: An Innovative Pathway for Acrylonitrile Derivatives. Catal Lett 146, 1687–1705 (2016). https://doi.org/10.1007/s10562-016-1793-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1793-7