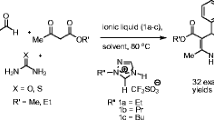
Abstract
Unprecedented examples of 1-alkyl-1,2,4-triazolium methanesulfonate based Brønsted acidic room temperature ionic liquids were synthesized and characterized. Their catalytic activity and efficiency in recyclability and reusability on one pot synthesis of halide substituted β-amino carbonyl compounds are reported.
Graphical Abstract



Similar content being viewed by others
Notes
The pH was measured using Digital pH meter Deepvision Model 111/101. All RTILs (5 mmol) were dissolved in triply distilled water (10 mL) for pH measurement. pH instrument was calibrated with buffer pH 2.0.
References
Meng X, Xiao SF (2014) Chem Rev 114:1521–1543
Federsel JH (2013) Green Chem 153:105–3115
Horvath T, Anastas PT (2007) Chem Rev 107:2167–2168
Pinaki LH, Bhadury S, Song B, Yang S (2012) RSC Adv 2:12525–12551
Hallett PJ, Welton T (2011) Chem Rev 111:3508–3576
Hajipouri R, Rafiee F (2009) J Iran Chem Soc 6:647–678
Welton T (1999) Chem Rev 99:2071–2083
Erdmenger T, Sanchez GC, Vitz J, Hoogenboom R, Schubert SU (2010) Chem Soc Rev 39:3317–3333
Greaves LT, Drummond JC (2008) Chem Rev 108:206–237
Fukaya Y, Iizuka Y, Sekikawa K, Ohno H (2007) Green Chem 9:1155–1157
Miao W, Chan HT (2006) Acc Chem Res 39:897–908
Wang R, Jin MC, Twamley B, Shreeve M (2006) J Inorg Chem 45:6396–6403
Dupont J (2004) J Braz Chem Soc 15:341–350
Crosthwaite MJ, Sudhir N, Aki KV, Maginn JE, Rennecke BF (2004) J Phys Chem B 108:5113–5119
Mirzaei RY, Twamley B, Shreeve MJ (2002) J Org Chem 67:9340–9345
Seddon RK, Stark A, Torres M (2000) Pure Appl Chem 72:2275–2287
Daily AL, Miller MK (2013) J Org Chem 78:4196–4201
Luo J, Hu J, Saak W, Beckhaus R, Wittstock G, Vankelecom JF, Agert C, Conrad O (2011) J Mater Chem Rev 21:10426–10436
Gnanamgari D, Moores A, Rajaseelan E, Crabtree HR (2007) Organometallics 26:1226–1230
Xue H, Twamley B, Shreeve MJ (2004) J Org Chem 69:1397–1400
Domling A, Wang W, Wang K (2012) Chem Rev 112:3083–3135
Gu Y (2012) Green Chem 14:2091–2128
Toure BB, Hall GD (2009) Chem Rev 109:4439–4486
Subramanipillai GS (2013) J Chem Sci 125:467–482
Azizi N, Torkiyan L, Saidi RM (2006) Org Lett 8:2079–2082
Sahoo S, Joseph T, Halligudi BS (2006) J Mol Catal A 244:179–182
Palaniappan S, John A, Amarnath A, Rao JV (2004) J Mol Catal A 218:47–53
Bur KS, Martin FS (2001) Tetrahedron 57:3221–3242
Pachamuthu PM, Shanthi K, Luqueand R, Ramanathan A (2013) Green Chem 15:2158–2166
Amene YA, Reza N-J, Ali Sharifi, Saeed A, Mojtaba M (2013) J Chem Res 37:216–218
Kumar A, Gupta KM, Kumar M (2012) Green Chem 14:290–295
Maria DP, Bracco P, Castelhano FL, Bargeman G (2011) ACS Catal 1:70–75
Feng CL, Sun WY, Tang JW, Xu JL, Lam LK, Zhou Z, Chan SA (2010) Green Chem 12:949–952
Kidwai M, Bhatanagar D, Mishra KN, Bansal V (2008) Catal Commun 9:2547–2549
Guoying Z, Tao J, Haixiang G, Buxing H, Jun H, Donghai S (2004) Green Chem 6:75–77
Haixiang G, Buxing H, Junchun L, Tao J, Zhimin L, Weize W, Yanhong C, Jianmin Z (2004) Synth Commun 34:3083–3089
Cai BY, Ting FY, Chuan BZ, Gang L (2009) J Ind Eng Chem 15:653–656
Elango K, Srirambalaji R, Anantharaman G (2007) Tetrahedron Lett 48:9059–9062
Anantharaman G, Elango K (2007) Synth React Inorg Met 37:719–723
Jizong L, Yanging P, Gonghua S (2005) Catal Lett 102:159–162
Fang D, Luo J, Zhou XL, Liu ZL (2007) Catal Lett 116:76–80
Sultan GE, Cinzia C, Zeynep T, Sunita R (2011) RSC Adv 1:761–764
Rajendra S (2010) Catal Lett 139:17–25
Caibo Y (2010) Synth Commun 40:3640–3647
Fang D, Fei Z, Liu Z (2009) Catal Lett 10:1267–1270
Nemati F, Fakhaei SA, Amoozadeh A, Hayeniaz SY (2011) Synth Commun 41:3695–3702
Nemati F, Bigdeli AM, Mahdavinia HG, Kiani H (2010) Green Chem Lett Rev 3:89–92
Boumoud B, Zetchi A, Boumoud T, Debache A (2012) J Chem Pharm Res 4:2517–2521
Hua L, Zeng YH, Shao WH (2009) Tetrahedron Lett 50:6858–6860
Ajeet A, Yelwande, Arbad RB, Lande KM (2011) J Korean Chem Soc 55:644–648
Kozlov SN, Isaeva KR (1965) Zh Obshch Khim 35:285–288
Kozlov SN, Nikolaev DA (1961) Zh Obshch Khim 31:3894–3896
Kidwai M, Mishra KN, Bansal V, Kumar A, Mozumda S (2009) Tetrahedron Lett 50:1355–1358
Abedini TJ, Eshghi H, Bakavoli M, Rahimizadeh M (2014) Res Chem Intermed 10:1–7
Acknowledgments
The authors thank the Department of Science and Technology—Science and Engineering Research Board (DST-SERB, SR/FT/CS-60/2011), India for funding. Also the authors thank Dr. G. Anantharaman, Department of Chemistry, Indian Institute of Technology Kanpur for ESI-MS measurement.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Data
ESI mass spectra and NMR for all ionic liquids are available.
Rights and permissions
About this article
Cite this article
Nagarajan, S., Kandasamy, E. Reusable 1,2,4-Triazolium Based Brønsted Acidic Room Temperature Ionic Liquids as Catalyst for Mannich Base Reaction. Catal Lett 144, 1507–1514 (2014). https://doi.org/10.1007/s10562-014-1312-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1312-7