Abstract
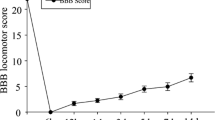
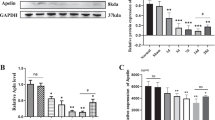
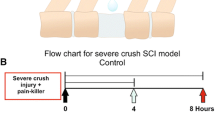
Neural progenitor cells (NPCs) transplantation is known as a potential strategy for treating spinal cord injury (SCI). This study aimed to investigate effects of insulin growth factor-1 (IGF-I) on NPCs proliferation and clarify associated mechanisms. NPCs isolated from T8-T10 segmental spinal cord tissues of rats were cultured and identification. Then, lentivirus packing plasmids containing IGF-I was constructed and used for NPCs infection. Cell proliferation was evaluated by detecting 5-Bromodeoxyuridine (BrdU) expression in NPCs, cell differentiation was detected using double-labeling immunofluorescence staining while cell apoptosis was detected using TUNEL assay. In addition, the signal expression of Akt/mTOR/p70S6K in NPCs cells were investigated using immunofluorescence staining and western blot assay. The experimental group was defined as pCMV-IGF-I group, while the negative control group was defined as pCMV-LacZ group. Cells infected with pCMV-IGF-I lentivirus followed by addition of 100 mg/ml rapamycin were defined as pCMV-IGF-I + Rapa group. NPCs were successfully isolated, identified and cultured. IGF-I overexpression significantly inhibited cell apoptosis and enhanced cell migration. Akt/mTOR/ p70S6K signaling cascade was proved to be present in NPCs, IGF-I overexpression significantly activated Akt/mTOR/p70S6K signaling cascade, while rapamycin addition inhibited its expression. Also, the activated Akt/mTOR/p70S6K signal cascade induced by IGF-I significantly enhanced BrdU expression and inhibited cell apoptosis, and promoted the differentiation of NPC into the neuronal system. However, the rapamycin addition inhibited the cell response induced by IGF-I overexpression. IGF-I overexpression could enhance cell proliferation, inhibit cell apoptosis and promote their differentiation into neuronal systems by activating Akt/mTOR/p70S6K signaling cascade in vitro, indicating that the Akt/mTOR/p70S6K signaling cascade may be the potentially mechanism for the endogenous repair and remodeling of spinal cord after injury.










Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Anjum A et al (2020) Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 21:7533
Ashammakhi N et al (2019) Regenerative therapies for spinal cord injury. Tissue Eng Part B-Re 25:471–491
Bhushan S et al (2007) A triterpenediol from boswellia serrata induces apoptosis through both the intrinsic and extrinsic apoptotic pathways in human leukimia HL-60 cells. Apoptosis 12:1911–1926
Chen A et al (2013) Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep 8:1011–1016
Cheng C, Dong W (2018) Aloe-emodin induces endoplasmic reticulum stress dependent apoptosis in colorectal cancer cells. Med Sci Monit 24:6331–6339
Cooper JE (2011) Anesthesia, analgesia and euthanasia of invertebrates. ILAR J 52:196–204
Costales J, Kolevzon A (2016) The therapeutic potential of insulin-like growth factor-1 in central nervous system disorders. Neurosci Biobehav R 63:207–222
Cummings BJ et al (2005) Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA 102:14069–14074
DiVall SA et al (2010) Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest 120(8):2900–2909
Du W et al (2018) The prognostic value of serum neuron specific enolase (NSE) and S100B level in patients of acute spinal cord injury. Med Sci Monit 24:4510–4515
Gan B et al (2008) mTORC1-dependent and independent regulation of stem cell renewal, differentiation and mobilization. Proc Natl Acad Sci USA 105:19384–19389
Gorris R et al (2015) Pluripotent stem cell-derived radial glia-like cells as stable intermediate for efficient generation of human oligodendrocytes. Glia 63:2152–2167
Harada H et al (2001) p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci 98(17):9666–9670
Hernandez J, Torres-Espin A, Navarro X (2011) Adult stem cell transplants for spinal cord injury repair: current state in preclinical research. Curr Stem Cell Res Ther 6:273–287
Ho SY et al (2017) SDF-1/CXCR4 signaling maintains stemness signature in mouse neural stem/progenitor cells. Stem Cells Int 2017:1–14
Hormigo KM et al (2017) Enhancing neural activity to drive respiratory plasticity following cervical spinal cord injury. Exp Neurol 287:276–287
Icli B et al (2019) MicroRNA-615-5p regulates angiogenesis and tissue repair by targeting AKT/eNOS (protein kinase B/endothelial nitric oxide synthase) signaling in endothelial cells. Arterioscl Throm Vas 39(7):1458–1474
Idda ML et al (2012) Circadian timing of injury-induced cell proliferation in zebrafish. PLoS ONE 7:e34203
Jiang L, Zhao YD, Chen WX (2017) The function of the novel mechanical activated ion channel piezo1 in the human osteosarcoma cells. Med Sci Monit 23:5070–5082
Jung HJ, Suh Y (2015) Regulation of IGF-1 signaling by microRNAs. Front Genet 5:472
Karimi-Abdolrezaee S et al (2006) Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci 26:3377–3389
Lane MA, Lepore AC, Fischer I (2017) Improving the therapeutic efficacy of neural progenitor cell transplantation following spinal cord inury. Expert Rev Neurother 17:433–440
Li X et al (2018) Curcumol induces cell cycle arrest and apoptosis by inhibiting IGF-1R/PI3K/Akt signaling pathway in human nasopharyngeal carcinoma NCE-2 cells. Phytother Res 32:2214–2225
Liu L et al (2019) Curcumin induces apoptotic cell death and protective autophagy by inhibiting AKT/mTOR/p70S6K pathway in human ovarian cancer cells. Arch Gynecol Obstet 299(6):1627–1639
Magistri M et al (2016) A comparative transcriptomic analysis of astrocytes differentiation from human neural progenitor cells. Eur J of Neurosci 44(10):2858–2870
Radisavljevic Z (2013) Akt as locus of cancer angiogenic robustness and fragility. J Cell Physiol 228:21–24
Ratajczak J et al (2011) Higher number of stem cells in the bone marrow of circulating low Igf-1 level laron dwarf mice-novel view on Igf-1, Stem cells and Aging. Leukemia 25:729–733
Salazar DL et al (2010) Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS ONE 5:e12272
Shin JC et al (2015) Clinical trial of human fetal brain-derived neural stem/prognitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast 2015:630932
Witiw CD, Fehlings MG (2015) Acute spinal cord injury. J Spinal Disord Tech 28(6):202–210
Yakar S et al (1999) Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci 96(13):7324–7329
Yang J et al (2018) Evaluation of hypoxia on the expression of miR-646/IGF-1 signaling in human periodontal ligament cells. Med Sci Monit 24:5282–5291
Yu M et al (2015) Insulin-like growth factor-1 (IGF-I) promotes myoblast proliferation and skeletal muscle growth of embryonic chickens via the PI3K/Akt signalling pathway. Cell Biol Int 39:910–922
Yu Y et al (2012) Insulin-like factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol 137:513–525
Yuan Y et al (2020) Effects of Akt/mTOR/p70S6K signaling pathway regulation on neuron remodeling caused by translocation repair. Front Neurosci 14:565870
Zhang F et al (2021) Decellularized nerve extracellular matrix/chitosan crosslinked by genipin to prepare a moldable nerve repair material. Cell Tissue Bank 4:1–12
Zhao SL et al (2014) Mesenchymal stem cells with overexpression of midkine enhance cell survival and attenuate cardiac dysfunction in a rat model of myocardial infarction. Stem Cell Res Ther 5:37
Zhu Y et al (2018) Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev Dyn 247:75–94
Ziv Y et al (2006) Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci USA 103:13174–13179
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant No. 81100929), Medical Research Innovation Project Plan of Sichuan Province (Grant No. Q15032) and Science & Technology Project of Nanchong City (18SXHZ0374).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no competing financial or commercial interests in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, B., Hu, L., Zhang, J. et al. Insulin growth factor-1 enhances proliferation and inhibits apoptosis of neural progenitor cells by phosphorylation of Akt/mTOR/p70S6K molecules and triggering intrinsic apoptosis signaling pathway. Cell Tissue Bank 23, 459–472 (2022). https://doi.org/10.1007/s10561-021-09956-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-021-09956-2