Abstract
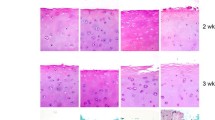
To compare the quality of the repair tissue in three-dimensional co-culture of human chondrocytes implanted in an in vivo model. Six cadaveric and five live human donors were included. Osteochondral biopsies from the donor knees were harvested for chondrocyte isolation. Fifty percent of cadaveric chondrocytes were expanded until passage-2 (P2) while the remaining cells were cryopreserved in passage-0 (P0). Fresh primary chondrocytes (P0f) obtained from live human donors were co-cultured. Three-dimensional constructs were prepared with a monolayer of passage-2 chondrocytes, collagen membrane (Geistlich Bio-Gide®), and pellet of non-co-cultured (P2) or co-cultured chondrocytes (P2 + P0c, P2 + P0f). Constructs were implanted in the subcutaneous tissue of athymic mice and left for 3 months growth. Safranin-O and Alcian blue staining were used to glycosaminoglycan content assessment. Aggrecan and type-II collagen were evaluated by immunohistochemistry. New-formed tissue quality was evaluated with an adaptation of the modified O’Driscoll score. Histological quality of non-co-cultured group was 4.37 (SD ±4.71), while co-cultured groups had a mean score of 8.71 (SD ±3.98) for the fresh primary chondrocytes and 9.57 (SD ±1.27) in the cryopreserved chondrocytes. In immunohistochemistry, Co-culture groups were strongly stained for type-II and aggrecan not seen in the non-co-cultured group. It is possible to isolate viable chondrocytes from cadaveric human donors in samples processed in the first 48-h of dead. There is non-significant difference between the numbers of chondrocytes isolated from live or cadaveric donors. Cryopreservation of cadaveric primary chondrocytes does not alter the capability to form cartilage like tissue. Co-culture of primary and passaged chondrocytes enhances the histological quality of new-formed tissue compared to non-co-cultured cells.








Similar content being viewed by others
References
Ahmed N, Gan L, Nagy A, Zheng J, Wang C, Kandel RA (2009) Cartilage tissue formation using redifferentiated passaged chondrocytes in vitro. Tissue Eng Part A 15:665–673
Baghaban Eslaminejad M, Taghiyar L, Falahi F (2009) Quantitative analysis of the proliferation and differentiation of rat articular chondrocytes in alginate 3D culture. Iran Biomed J 13:153–160
Bian L, Zhai DY, Mauck RL, Burdick JA (2011) Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A 17:1137–1145
Casper ME, Fitzsimmons JS, Stone JJ, Meza AO, Huang Y, Ruesink TJ, O’Driscoll SW, Reinholz GG (2010) Tissue engineering of cartilage using poly-epsilon-caprolactone nanofiber scaffolds seeded in vivo with periosteal cells. Osteoarthritis Cartilage 18:981–991
Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG (1997) Cartilage injuries: a review of 31,516 knee arthroscopies. Arthrosc J Arthrosc Relat Surg 13:456–460
Farr J, Cole B, Dhawan A, Kercher J, Sherman S (2011) Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res 469:2696–2705
Gartland A, Mechler J, Mason-Savas A, MacKay CA, Mailhot G, Marks SC Jr, Odgren PR (2005) In vitro chondrocyte differentiation using costochondral chondrocytes as a source of primary rat chondrocyte cultures: an improved isolation and cryopreservation method. Bone 37:530–544
Giovannini S, Diaz-Romero J, Aigner T, Heini P, Mainil-Varlet P, Nesic D (2010) Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cells Mater 20:245–259
Hamada T, Sakai T, Hiraiwa H, Nakashima M, Ono Y, Mitsuyama H, Ishiguro N (2013) Surface markers and gene expression to characterize the differentiation of monolayer expanded human articular chondrocytes. Nagoya J Med Sci 75:101–111
Hjelle K, Solheim E, Strand T, Muri R, Brittberg M (2002) Articular cartilage defects in 1,000 knee arthroscopies. Arthrosc J Arthrosc Relat Surg 18:730–734
Hunziker EB (2002) Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10:432–463
Ibarra C, Izaguirre A, Villalobos E, Masri M, Lombardero G, Martinez V, Velasquillo C, Meza AO, Guevara V, Ibarra LG (2014) Follow-up of a new arthroscopic technique for implantation of matrix-encapsulated autologous chondrocytes in the knee. Arthrosc J Arthrosc Relat Surg 30:715–723
Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, Madry H, Mata A, Mauck RL, Semino CE, Stoddart MJ (2013) Tissue engineering for articular cartilage repair—the state of the art. Eur Cells Mater 25:248–267
Juhasz T, Matta C, Somogyi C, Katona E, Takacs R, Soha RF, Szabo IA, Cserhati C, Szody R, Karacsonyi Z, Bako E, Gergely P, Zakany R (2014) Mechanical loading stimulates chondrogenesis via the PKA/CREB-Sox9 and PP2A pathways in chicken micromass cultures. Cell Signal 26:468–482
Kolettas E, Muir HI, Barrett JC, Hardingham TE (2001) Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of Sox-9 transcription factor. Rheumatology 40:1146–1156
Masri M, Lombardero G, Velasquillo C, Martinez V, Neri R, Villegas H, Ibarra C (2007) Matrix-encapsulation cell-seeding technique to prevent cell detachment during arthroscopic implantation of matrix-induced autologous chondrocytes. Arthrosc J Arthrosc Relat Surg 23:877–883
McNickle AG, Provencher MT, Cole BJ (2008) Overview of existing cartilage repair technology. Sports Med Arthrosc Rev 16:196–201
Negri S, Fila C, Farinato S, Bellomi A, Pagliaro PP (2007) Tissue engineering: chondrocyte culture on type 1 collagen support. Cytohistological and immunohistochemical study. J Tissue Eng Regen Med 1:158–159
Olivos-Meza A, Fitzsimmons JS, Casper ME, Chen Q, An KN, Ruesink TJ, O’Driscoll SW, Reinholz GG (2010) Pretreatment of periosteum with TGF-beta1 in situ enhances the quality of osteochondral tissue regenerated from transplanted periosteal grafts in adult rabbits. Osteoarthritis Cartilage 18:1183–1191
Oseni AO, Butler PE, Seifalian AM (2013) Optimization of chondrocyte isolation and characterization for large-scale cartilage tissue engineering. J Surg Res 181:41–48
Qing C, Wei-ding C, Wei-min F (2011) Co-culture of chondrocytes and bone marrow mesenchymal stem cells in vitro enhances the expression of cartilaginous extracellular matrix components. Braz J Med Biol Res 44:303–310
Tew SR, Murdoch AD, Rauchenberg RP, Hardingham TE (2008) Cellular methods in cartilage research: primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods 45:2–9
Wei Y, Zeng W, Wan R, Wang J, Zhou Q, Qiu S, Singh SR (2012) Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. Eur Cells Mater 23:1–12
Widuchowski W, Widuchowski J, Trzaska T (2007) Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 14:177–182
Acknowledgements
This work is supported by the Mexican Council of Science (CONACyT) Grant SALUD-PDCPN-2013-01-215138; Technology, Science and Innovation Secretary Grants: SECITI 079 BIS/2013 and SECITI/INR/GOB-25/2013. The authors wish specialty to thank to Biograft of México Musculoskeletal Tissue and Skin Bank; Blood Bank & Pathology Service at the National Institute of Rehabilitation; and Autonomous National University of Mexico (UNAM). We also appreciate the support and collaboration of Veterinary Facility at the National Institute of Rehabilitation: Hugo-Lecona DMV and Javier-Perez DMV.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Olivos-Meza, A., Velasquillo Martínez, C., Olivos Díaz, B. et al. Co-culture of dedifferentiated and primary human chondrocytes obtained from cadaveric donor enhance the histological quality of repair tissue: an in-vivo animal study. Cell Tissue Bank 18, 369–381 (2017). https://doi.org/10.1007/s10561-017-9635-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-017-9635-4